Antibiotic resistance in Italy:
latest evidence
In nature, bacteria have one or more resistance genes in their genetic complement, which allow the development of specific defence mechanisms towards one or more classes of antibiotics, decreasing or deleting their effectiveness (or, better to say, the sensitivity to they). Therefore, antimicrobial resistance (AMR) represents a natural evolutionary process. It can undergo an increase with the improper and/or excessive use of antimicrobials: this selective pressure benefits the exchange of genetic material with other bacteria that may be present (pathogens and commensals) and the transmission of resistance. Over the years, numerous actions have been taken to reduce the consumption of antimicrobials in the veterinary field, at multiple levels (diagnostics, monitoring, pharmaco-surveillance, training, communication, research), although the problem of AMR persists.
The European Food Safety Authority (EFSA), with its annual reports, monitors the European situation on the trends and prevalence of antibiotic resistance in humans, animals for food production (DPA) and food of animal origin, harmonizing the data to the Epidemiological Cut-off values (ECOFFs). They don’t suffer alterations related to the frequency of administration of antimicrobials, allowing better comparability and interpretability of the data: an isolate, therefore, will be considered resistant if the minimum inhibitory concentration (MIC) is > ECOFF. The level of resistance of the indicator bacterium to the main antimicrobial molecules is attributed on the basis of well-defined percentage ranges, attributable to the percentage of resistant isolates compared to the total number of isolates tested of that microorganism in that country. EFSA and the European Center for Disease Prevention and Control (ECDC) have established zoonotic bacteria as "antimicrobial resistance indicators" for humans, animals and food:
- Campylobacter spp.
- Escherichia coli
- Salmonella spp.
- Staphylococcus aureus-meticillino-resistente (MRSA)
- enterobatteri produttori di:
1) β-lattamasi di tipo ESBL (in grado di trasmettere la resistenza alle penicilline e alle cefalosporine, dalla prima alla quarta generazione, ma sono tuttavia inibite dall’acido clavulanico)
2) β-lattamasi di tipo AmpC (le quali sono una causa comune di resistenza alle cefalosporine dalla prima alla terza generazione e non possono essere inibite dall’acido clavulanico)
3) carbapenemasi (con conseguente spiccata multi-resistenza a tutti gli antibiotici β-lattamici, ovvero penicilline, cefalosporine, monobattami e carbapenemi).
- The high prevalence of E. coli with ESBL+ phenotype in calves (< 1 year) (98.5%), a similar figure in beef (93.8%), with increasing presence of ESBL+ isolates co-resistant to clavulanic acid and cephalosporin third generation ceftriaxone in beef (37.5%). Also in the Italian pig and poultry sectors the prevalence of ESBL producers is at extremely high levels (pork: 88.2%; fattening-pigs: 85%; broilers and chicken meat: 79.5%). On the other hand, there isa low prevalence of AmpC+ and AmpC+/ ESBL+ phenotypes in compartments of Italian livestock production (Table 1).
Table 1. Difference in the prevalence of E. coli isolates with ESBL+, AmpC+, ESBL+/AmpC+, CP+ and ESBL+ phenotype resistant to CLA/CTX in the different sources in Italy in 2019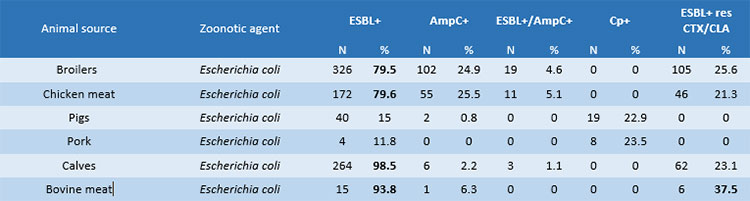
- Regarding the prevalence of resistance to critical antimicrobials (Table 2), Salmonella Kentucky (100%) and Salmonella Infantis (95%) acquired greater resistance in turkeys and broilers, reaching saturated levels against quinolones and fluoroquinolones; results are lower but still high for the same classes in Campylobacter jejuni isolates (64% and 87%) and Escherichia coli (53.4% and 64%) in chicken carcasses. In calves, there is an increase in C. jejuni isolates resistant to quinolone nalidixic acid (68.9%) and fluoroquinolone ciprofloxacin (69.8%) and E. coli isolates resistant to ciprofloxacin (46.2 %). There is a better situation regarding Salmonella spp. and E. coli in Italian pig farming. Finally, in egg production, Salmonella Kentucky, as in broiler chicken, is the serovar that has acquired critical resistance levels (95.2%), toboth nalidixic acid and ciprofloxacin. The Salmonella Enteritidis serotype, on the other hand, shows a worrying level of resistance to colistin (38.2%).
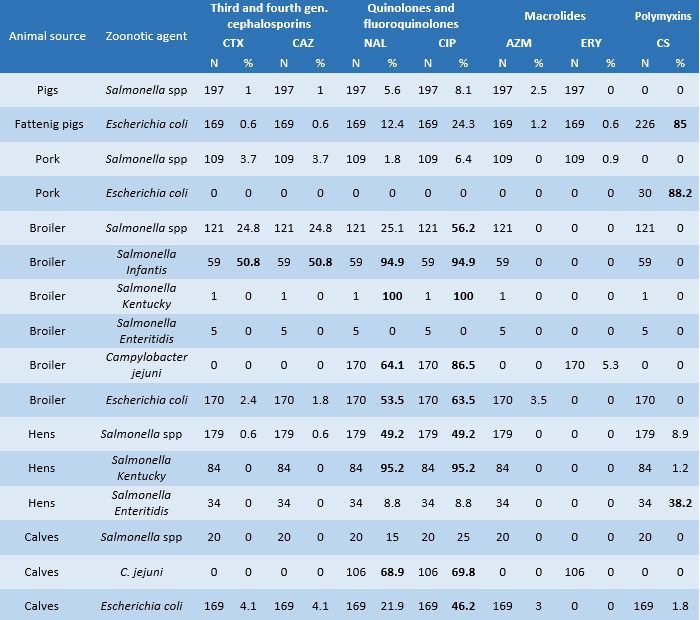
-
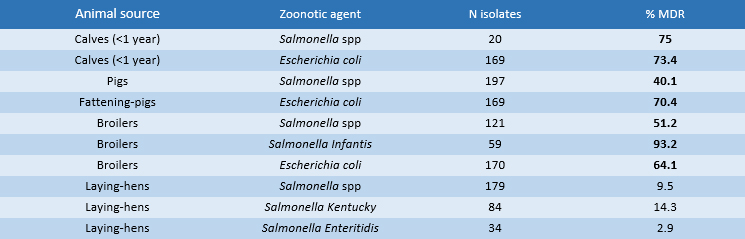
Regarding multi-drug-resistance (MDR) (i.e. strains not susceptible to at least three classes of antimicrobials) (Table 3) in zoonotic strains isolated in calves, there is an extremely high prevalence of the MDR+ phenotype in Salmonella spp. (75%) and in Escherichia coli (73.4%). In fattening-pigs, the prevalence of MDR+ phenotypes in E. coli isolates is 70.4% while in Salmonella spp. the MDR level is 40.1%. In both livestock sectors, cross-resistance concerns ampicillin, sulfamethoxazole and tetracycline. In broilers, Salmonella Infantis predominates with a resistance level of 93.2% while lower levels are recorded for the MDR+ isolates of E. coli (64%) and Salmonella spp. (51%). Multi-resistance concerns ampicillin, ciprofloxacin, sulfamethoxazole, trimethoprim and tetracycline. There are optimistic results in the laying hens sector as MDR+ levels are not reached i.e. resistance for each indicator species does not cover more than two antimicrobial classes: Salmonella spp. and Salmonella Kentucky reach high resistance levels only for quinolones and fluoroquinolones 49.2% and 95.2% in both categories, respectively. The serovar Salmonella Enteritidis shows high resistance only to polymyxins (38.2%).
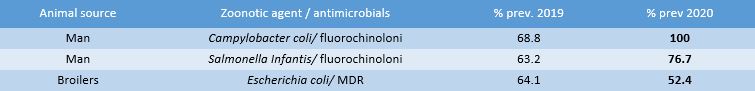
At the end of March 2022, the new EU summary report on antimicrobial resistance in zoonotic bacteria and indicators of humans, animals and food [6] was published, relating to the two-year period 2019-2020, from which an almost constant national picture emerged, with differences in prevalence rates that do not exceed a few units, except for the following bacterial-matrix combinations indicative of the risk of antimicrobial resistance [7]:
- an extremely significant upward trend in resistance to fluoroquinolones for Campylobacter coli in humans, up to saturation levels (in 2019 it was 68.8% while in 2020 it was 100%);
- an upward trend in resistance to fluoroquinolones also in national isolates of Salmonella Infantis in humans (from 63.2% to 76.7%);
- a decline in the prevalence of E. coli multidrug resistance in broilers (2019: 64.1%; 2020: 52.4%) (Table 4).
Table 4. Difference in the prevalence of isolates with resistant phenotype, by zoonotic species, in Italy in 2020 compared to 2019
On the European Surveillance Project on the Consumption of Veterinary Antimicrobials (ESVAC), the latest report [3], published in November 2021, based on the harmonized data of the annual sales of the Member States and on the estimate of the entire animal population present in the year of observation in each country, highlights how the national trend is in sharp decline: in 2020 the sales of antibiotics in oral form were equal to 7.4 tons (t) while sales in other pharmaceutical forms 689.3 t, for a total of 696.7 t, with a decrease of 43% compared to the starting figure of 2016 ( 1213.2 t). The total sale of antimicrobial agents in DPAs reached 181.8 mg/PCU in 2020: the main best-selling classes remain penicillins and tetracyclines (33.6% and 26.9%, respectively, of total sales in 2020). Another encouraging result is the reduction in the sale of antimicrobials considered of critical importance and included in the B list of the AMEG categorization of the European Medicines Agency (EMA) (Figure 1) [4], and those considered to be of the highest priority for humans by the World Health Organization (WHO):
- 0,70 mg/PCU for polymyxins, lower than the European average of 2,58 mg/PCU, which represented only 0.4% of the total sales;
- 0,16 mg/PCU for third and fourth generation cephalosporins, in line with the European average, which represented only 0.4% of the total sales;
- 1,23 mg/PCU for fluoroquinolones, lower than the European average of 2.20 mg/PCU, the sale of which represents 0.68% of the total;
- 0,78 mg/PCU for the other quinolones, however, it appears to be above the European average level of 0.73 mg/PCU;
- 8,68 mg/PCU against the European average of 16.55, with a percentage of sales on the total of 4.8% [5].
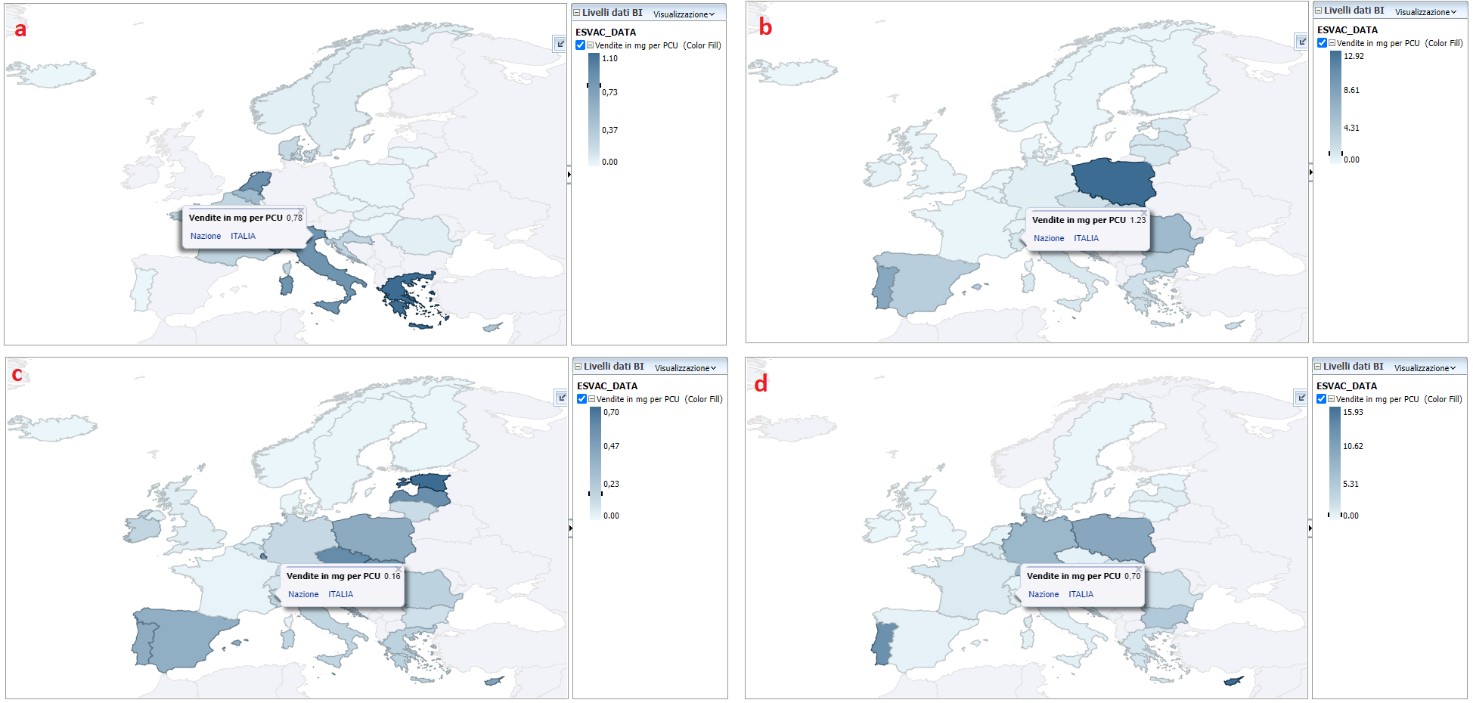
Figure 1. Territorial distribution of sales of quinolones (a), fluoroquinolones (b), third and fourth generation cephalosporins (c) and polymyxins (d) for food producing animals, in mg / PCU, in Italy, for 2020 [4]
References
- Pozio E., Murrell K. 2006. Systematics and epidemiology of Trichinella. Adv Parasitol., 63:367-439
- Pozio E. 2019. Trichinella and Trichinellosis in Europe. Veterinarski Glasnik, 00, 1-20
- Badagliacca P., Di Sabatino D., Salucci S., Romeo G., Cipriani M., Sulli N., Dall'Acqua F., Ruggieri M., Calistri P., Morelli D. 2016. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol., 231:124-127
- Remonti L., Balestrieri A., Domenis L., Banchi C., Lo Valvo T., Robetto S., Orusa R. 2005. Red fox (Vulpes vulpes) cannibalistic behaviour and the prevalence of Trichinella britovi in NW Italian Alps. Parasitol. Res., 97:431-435
- Commissione Europea. 2015. Regolamento di esecuzione (UE) 2015/1375 della Commissione del 10 agosto 2015 che definisce norme specifiche applicabili ai controlli ufficiali relativi alla presenza di Trichine nelle carni. Gazzetta Ufficiale, L 212, 7-34
- Kapel, C.M.O., 2005. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol., 132, 101-10.
- European Food Safety Authority, & European Centre for Disease Prevention and Control, [Online]
- Autorità europea per la sicurezza alimentare e Centro europeo per la prevenzione e il controllo delle malattie. (2022). Relazione di sintesi dell'Unione europea sulla resistenza antimicrobica nei batteri zoonotici e indicatori provenienti da esseri umani, animali e alimenti nel 2019/2020 [set di dati].
Lilla Vizza
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale" (IZSAM)