Epidemiological situation of lumpy skin disease in Europe and neighbouring Countries
The disease
Lumpy skin disease (LSD) is an economically important disease of cattle transmitted by a poxvirus, the lumpy skin disease virus (LSDV), a member of the genus Capripoxvirus within the Poxviridae family causing characteristic skin lesions.
LSD is a disease with high morbidity and low mortality rate affecting cattle of all ages and breeds. LSD is host specific causing natural infection in cattle and Asian water buffalo (Bubalus bubalis) although the mortality is significantly lower in buffalo (1.6%) than in cattle (30.8%) (El-Nahas E.M. et al., 2011) (Tuppurainen E. et al., 2017).
LSDV causes a very characteristic and easily recognizable clinical disease. After an initial period of fever and lymphadenopathy the animal develops large, firm cutaneous nodules up to 5 cm in diameter. These can be found all over the body but particularly on sparsely haired areas such as the head, udder, scrotum and perineum (Beard P.M., 2016).
Generally, direct contact has been shown to be an ineffective route for the transmission of LSDV, but actual experimental evidence is scarce. To date, only mechanical transmission is implicated for LSDV.
In severely infected animals, skin lesions are known to contain high titers of virus, providing a plentiful source of contamination for biting and blood-feeding arthropods.
Open skin lesions and ulcers offer an attractive source of nutrients for flies. Infectious LSD viruses are known to survive in skin lesions for at least 39 days post-infection.
The common stable fly (Stomoxys calcitrans), with a global distribution, is the most widely suspected vector species for LSDV spread. Stable flies are aggressive and persistent feeders and, since their bites are painful, feeding is often interrupted by the host, requiring flies to continue on another host. Thus, stable flies usually require three to five feeding attempts to achieve satiety.
Mechanical transmission of LSDV from infected to naïve hosts has
Been experimentally demonstrated in Rhipicephalus appendiculatus (Tuppurainen et al., 2013) and Amblyomma hebraeum (Lubinga et al.,2013) male ticks. The presence of LSDV has been demonstrated in tick saliva after feeding on infected cattle (Lubinga et al., 2013), and transstadial transmission of the virus has also been reported (Lubinga et al., 2014b).
Epidemiology history
The disease was firstly reported in Zambia in 1929. Until 1984, LSDV was maintained within the countries of sub Saharan Africa where its pathogenicity increased over time leading to severe pandemics. From 1984 the disease made several transcontinental jumps. The first transcontinental spread was confirmed in 1989 in Israel, when the disease spread from African to Asian Middle East countries, probably attributed to wind-borne transmission via stable flies (Stomoxys calcitrans) from Egypt. Despite the vaccination of cattle in both Egypt and Israel, with sheep poxvirus to control the infection, LSD cases re-emerged in Egypt and Israel in 2006 (Tuppurainen et al., 2017; Alkhamis et al., 2016; Awad W.S. et al., 2010). After these outbreaks and Syrian conflict, the LSD virus spread to Middle East countries and Turkey.
From 2015 to 2016, the disease spread from the western part of Turkey through eight Balkans countries (Albania, Montenegro, Serbia, Greece, Bulgaria and Macedonia) and to the other side in the Russian Federation. Starting from 2017 outbreaks declines in the Balkans until they disappear in 2018 thanks to the vaccination with LSD homologous strain and the control measures put in place in the region. In 2018, outbreaks were only reported in Russia (63), Turkey (46) and Georgia (6). (EFSA Journal 2019, Scientific report on lumpy skin disease: III).
Recently LSD cases have been reported in Russia (OIE, 2016; Lebedev N., 2016), Kazakhstan (OIE, 2016), in Armenia, Iran, Azerbaijan, Georgia and Balkan countries like posing emerging risk to Europe and other countries (Tuppurainen et al., 2017).
Epidemiology today
The last and third EFSA report on LSD, published on February 29 2019, provides a description of the epidemiological situation of LSD (Figure 1) and the vaccination campaign in place in Europe (Figure 2 and 3) and in neighbouring countries, such as Turkey and Russian Federation up to the end of 2018.
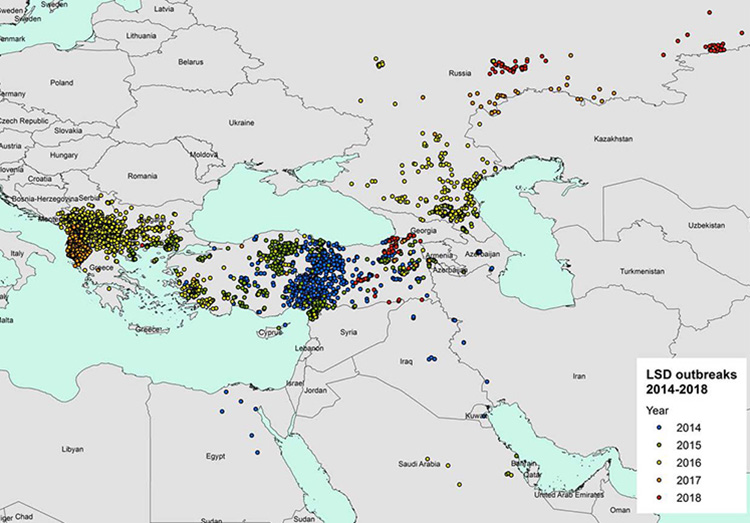
Figure 1. LSD outbreaks notified in Europe and Middle East between 2014 and 2018 (Data source: national authorities and ADNS for those countries that notify to ADNS and Empres-I for the other countries) (EFSA, Scientific report on lumpy skin disease: III. Data collection and analysis)
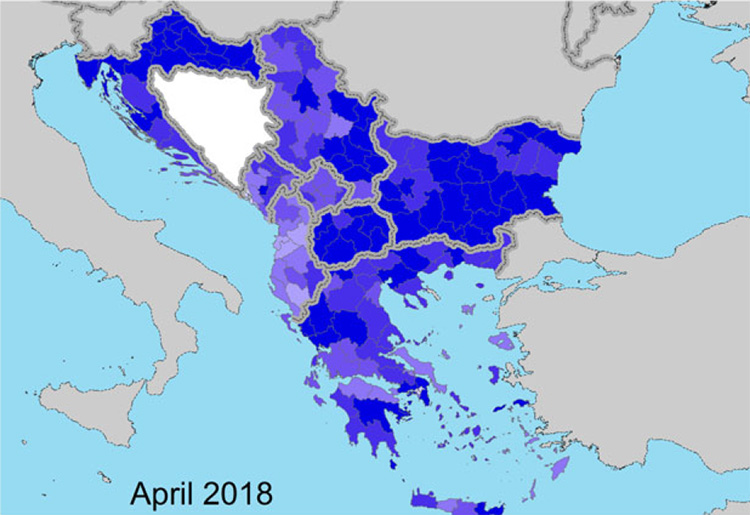
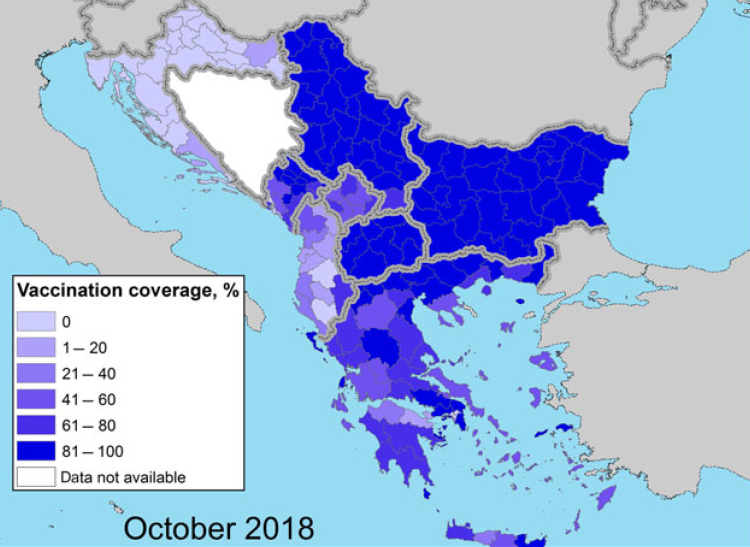
Figure 2 and 3. Vaccination coverage achieved in 2018 (proportion of immunised animals out of the total animals present) in the Balkan region at the beginning (April) and at the end (October) of the vector season (EFSA, Scientific report on lumpy skin disease: III. Data collection and analysis)
As you can find in the report “In 2018 no outbreaks of LSD were reported in the Balkan region after a decline in the number of outbreaks reported in 2017 (385) compared to 2016 (7,483). This confirms the effectiveness of the vaccination campaign based on the LSD homologous strain and the coordinated control measures put in place in the region. The halt of the LSD epidemic achieved in the Balkans in 2018 is in agreement with the long-term strategic objective of the LSD vaccination exit strategy in the South-Eastern Europe, which is to restore the LSD-free status situation as it was before the occurrence of the LSD epidemics and to stop the LSD vaccination in the region.
In 2018, LSD outbreaks were only reported in Russia (63 outbreaks), Turkey (46 outbreaks) and Georgia (6 outbreaks) in the period between April and November, thus confirming the seasonal pattern of LSD. Compared to 2017, the LSD epidemics in the Russian Federation expanded northward and eastward along the border with Kazakhstan, while in Turkey, most outbreaks were reported in the Eastern regions. The outbreaks in Turkey represent a threat for the neighbouring EU countries, especially Greece and Bulgaria, which can be at risk of new incursion”.
In a recent interesting study published in 2019 climatic variables, land cover and cattle density have been demonstrated by modelling to be positively associated to the spatial distribution of LSD outbreaks in an extensive area. The results of the model point out the importance of the temperature and the temperature range as predictors of LDS outbreaks, with higher values increasing the risk ok LSD outbreaks, land covered with croplands had 2.1 (95% CR: 1.2–2.5) times the odds of an LSD outbreak in forested areas. This likely due also to the vectors survival conditions but there could be other explanations like proximity with urban area which facilitates surveillance. Nevertheless the possibility of long jumps due to the movement of infected cattle remains, meaning that also countries far away from the active epidemic foci are at risk. (Allepuz A., Casal J. and Beltran-Alcrudo D., 2019).
The vaccination campaign
EFSA studied the effects of vaccination applied with different strategies (none, started after disease incursion, completed before disease incursion) combined with the partial compared with total stamping out in the infected herd. The preventive vaccination resulted to be the most effective strategy to eradicate the disease and the partial (i.e. of the infected animals or total stamping out (after detection of an infected case) results at this point to have the same effectiveness.
Two types of vaccines have been used in the affected countries: the heterologous derived from sheep and goat poxviruses and the live-attenuated homologous. Starting from 2016 mass vaccination campaigns with live homologous vaccines against LSD were carried out at regional level in south-eastern Europe in all affected countries and Croatia. High levels of median vaccination’s effectiveness (over 70 %) supported the protective effect of the vaccination in these countries. In 2018 no outbreaks were reported in those countries where vaccination campaign took place since 2016, after the disease enter, with live attenuated homologous strain as in Croatia and Bosnia and Herzegovina where a preventive vaccination took place. In 2018, new outbreaks have instead emerged in Russia, Turkey and Georgia demonstrating a tendency to move eastward and northward. In these countries a heterologous strain (Sheep and Goat Pox Virus vaccine) has been used, mostly as reactive vaccination (i.e. vaccination implemented after the disease has entered in a region) in the affected areas, achieving a coverage of around 70%. In Georgia, which experienced the disease since 2016, the vaccination has been put in place as preventive measure since 2014, after LSD outbreaks were detected in neighbouring Azerbaijan by creating a buffer zone of at least 10 km along the borders with the aforementioned country. Studies demonstrated that the Neethling attenuated vaccines successfully utilized in the Balkans is more effective than the attenuated sheep pox vaccines used in Turkey, Russian Federation and Georgia (Ben-Gera et al., 2015).
Transient decline in milk production, lump at the site of injection, loss of appetite and swelling are the symptoms reported in the field after vaccination. Again, field experience indicates that animals usually do not show any adverse reaction after the second injection. In Croatia and Bosnia and Herzegovina where large-scale preventive vaccinations were carried out in 2016 and 2017 was possible to obtain further information on the side effects without interference by the field strain.
Initial data reported by Croatian VAs through pharmacovigilance system showed postvaccination adverse reactions in 0.19% of the vaccinated farms, in 0.09% of the vaccinated animals with 0.02% deaths (EFSA, 2017).
The majority of symptoms were reported within 2 weeks after vaccination and included fever, decrease in milk production and oedema at the injection site (EFSA 2018). Nevertheless, these reports are based on case series without proper controls so that it is not possible properly estimate the production losses (milk yield, mortality, morbidity, etc.).
Conclusions
Vaccination and selective slaughter of infected animals has proved to be effective measures to control the disease. In particular, preventive vaccination and the use of the homologous vaccine should be encouraged. The movement of infected animals outside official controls remains the most difficult aspect of management and should always be taken into account when new outbreaks appear far from existing outbreaks.
References
- Alkhamis M.A. and VanderWaal K. (2016). Spatial and Temporal Epidemiology of Lumpy Skin Disease in the Middle East, 2012–2015. Front. Vet. Sci. 3:19. doi: 10.3389/fvets.2016.00019
- Allepuz A, Casal J, Beltrán-Alcrudo D. Spatial analysis of lumpy skin disease in Eurasia— Predicting areas at risk for further spread within the region. Transbound Emerg Dis. 2019; 66:813–822
- Awad W.S., Ibrahim A.K., Mahran K., Fararh K.M., Moniem MIA (2010). Evaluation of different diagnostic methods for diagnosis of lumpy skin disease in cows. Trop Anim Health Prod 42(4): 777-783
- Beard P.M. 2016. Lumpy skin disease: a direct threat to Europe. Veterinary Record May 28, 2016. doi: 10.1136/vr.i2800
- Ben-Gera, J., Klement, E., Khinich, E., Stram, Y., & Shpigel, N. Y. (2015). Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep‐pox live attenuated vaccines for the prevention of lumpy skin disease – The results of a randomized controlled field study. Vaccine, 38, 4837–4842
- EFSA (European Food Safety Authority), Calistri P, DeClercq K, Gubbins S, Klement E, Stegeman A, Corti~nas Abrahantes J, Antoniou S-E, Broglia A and Gogin A, 2019. Scientific report on lumpy skin disease: III. Data collection and analysis. EFSA Journal 2019;17(3):5638, 26 pp.
- El-Nahas E.M., El-Habbaa A.S., El-Bagoury G.F. and Radwan M.E.I. 2011. Isolation and identification of lumpy skin disease virus from naturally infected buffaloes at Kaluobia, Egypt. Global Veterinaria, 7: 234-237
- Lebedev, N. (2016). Standing Group of Experts on Lumpy Skin Disease in Europe under the GF-TADs Umbrella
- Lubinga, J.C., Tuppurainen, E.S.M., Stoltsz, W.H., Ebersohn, K., Coetzer, J.A.W., Venter, E.H., 2013. Detection of lumpy skin disease virus in saliva of ticks fed on lumpy skin disease virus-infected cattle. Exp. Appl. Acarol. 61, 129–138
- Lubinga, J.C., Tuppurainen, E.S.M., Coetzer, J.A.W., Stoltsz, W.H., Venter, E.H., 2014. Evidence of lumpy skin disease virus over-wintering by transstadial persistence in Amblyomma hebraeum and transovarial persistence in Rhipicephalus decoloratus ticks. Exp. Appl. Acarol. 62, 77–90
- Sprygina A.,Pestovaa Y. , Wallaceb D.B., Tuppurainena E., Kononova A.V. Transmission of lumpy skin disease virus: A short review
- Tuppurainen, E.S., Lubinga, J.C., Stoltsz, W.H., Troskie, M., Carpenter, S.T., Coetzer, J.A., Venter, E.H., Oura, C.A., 2013. Mechanical transmission of lumpy skin disease virus by Rhipicephalus appendiculatus male ticks. Epidemiol. Infect. 141, 425–430
- Tuppurainen E., Alexandrov T. & Beltrán-Alcrudo D. 2017. Lumpy skin disease field manual – A manual for veterinarians. FAO Animal production and Health Maual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 60 pages.
Valentina Zenobio
Centro Operativo Veterinario per l'Epidemiologia, Programmazione,
Informazione e Analisi del Rischio
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale"