Big data and vector-borne diseases
Vector-Borne Diseases (VBD) have always been a global health problem and an ongoing public health challenge. In the European Union, they are considered emerging infectious diseases whose incidence and geographical spread is rapidly increasing.
Climate and climate change play an important role in the spread of VBD by influencing their persistence in new areas, with direct effects on the reproduction of vertebrate vectors and hosts, which inevitably affect the rate of reproduction of pathogens. In addition, the impact of climate change indirectly causes changes in environmental ecology, also linked to the action of human activities, one of the most significant factors in the transmission of diseases with effects in the short to medium term.
To understand the epidemiology of VBD and the complex interactions between man-animals-environment, it is necessary to ensure a multidisciplinary and integrated approach in the control and prevention of these diseases, taking into account the complexities of existing interconnections. The era of Big data allows to approach a holistic view of VBD. In fact, effective data management in this area is, , a key element in improving the capabilities of the Arbovirosis early warning system. The processing and analysis of the information obtained allow to detect changes in the spatial distribution of diseases, and thus create real-time models to detect promptly new epidemics.
Address the issue with a One Health approach
Given the epidemiology of zoonoses and the complex interactions that develop between humans, animals and the environment, preventive medicine cannot be the only effective method of managing such events. In order to set up joint actions that respond to the challenges and objectives of the One Health approach, a shared strategic approach, allowing rapid responses and targeted adaptations in the field of animal disease prevention and surveillance, is essential.Integrating veterinary surveillance (animal and entomological) with that of human cases is essential to detect in advance vector circulation on the territory and to assess the risk of transmission of disease to humans; these choices can no longer have a national horizon only, but can be shared in policies for the protection of European public health.
Over the last few years, various control strategies have been implemented to strengthen the surveillance and monitoring of vectors at a global level among the major world organisations, in order to deal with possible epidemic emergencies in a coordinated manner. The World Health Organization (WHO) has developed the Global Vector Control Response (GVCR) 2017-2030, a project that aims at the development and integrated use of technologies for vector control and disease management, through the analysis of data whose use is of potential importance and value for public health. Moreovr, it provides thus providing partner countries with new strategies for action on vector control to prevent diseases and to
respond to new epidemics.
An innovative key to understanding expressed in the GVCR is the behavioural change to be implemented towards VBD, through an assessment of the resilience of the health system. The concept of resilience, which has been used in both social and environmental vocabulary, is a synthetic but very complex expression.
In the field of public health, the WHO develops this concept by working with partner countries to improve specific control and management measures against vector diseases according to the nation's eco-epidemiological and economic context. Furthermore, it permits to fulfill the implementation of individual good practices of VBD prevention, helping the individual to develop a greater capacity to manage diseases, so as to protect himself, but especially the community, from mosquitoes, ticks, flies and other vectors of diseases.
Among the other European projects, created with the aim of strengthening collaboration and communication between experts and organisations in the medical and veterinary sector with a view to a global approach to vector-borne diseases, the European Centre for Disease Prevention and Control (ECDC) and the European Food Safety Authority (EFSA) have also worked on a joint project. In 2017, a European network was set up with VectorNEt to exchange data on the geographical distribution of arthropod vectors, agents of human and animal diseases, with the aim of contributing to improving the responsiveness of the risk of introduction of vector-borne diseases into the EU).
A holistic approach to the issue
Although climate and climate change play an important role in influencing the spread of vector-borne diseases, the resurgence of some diseases cannot be attributed exclusively to it. Often the appearance of diseases follows ecological changes caused by human activities, and their distribution is determined by complex demographic, environmental and social factors. Changes in temperature and precipitation have changed the main structural features of agriculture and agricultural practices, also in response to increased trade and global trade. In many developing countries, unplanned urbanisation, which is the cause of deforestation and the consequent demographic development of population centres, has led to the growth of urban slums, providing a favourable habitat for the development of emerging diseases.
War conflicts have caused a worsening of the socio-economic conditions of the affected states, with the reduction of resources dedicated to public health. Human migration from war zones increased the risk of importing pests and vectors; this quickly led to the reappearance of diseases that were previously thought to have been eradicated. The growth of air traffic and the ever-increasing demand for the reduction of global transit in the short term for commercial purposes is another element that has played in favor of the transmission of vectors and related diseases, increasing the likelihood of their spread to other continents (Figure 1).
The complexity of the interaction of these multiple variables associated with the ecology and behaviour of the vector, but also those of the hosts and their immune status, presuppose the need for a holistic approach to vector-borne diseases, in which the need to operate on multiple levels of scientific knowledge is recognized, so as to produce a real state of control over their course.

Figure 1. Lines show direct links between airports, and the colour indicates passenger capacity in people per day (thousands [red]; hundreds [yellow]; tens [blue]). Routes linking regions at similar latitudes (in the northern or southern hemisphere) represent pathways that pathogens can move along
to reach novel regions. (Hufnagel et al.)
From the reds model to the predictive geoinformatics: the Big data a support of arbovirosis
For Ronald Ross, the Anglo-Saxon doctor who dedicated his entire career to the study of malaria, the "Great Malaria Problem" was mainly linked to an understanding of the causes linked to its transmission. The complex interaction between human mosquitoes and plasmodium that generates malaria was first modeled in the so-called "Ross Model" and is an example of how for more than a century attempts have been made to better understand diseases transmitted by vectors through the use of mathematical models.
The digital technologies developed in recent years have allowed the production and storage of a growing volume of data, the Big Data, which with the advent of social media and the ease of real-time data acquisition have allowed to establish connections between different sectors with which in the past it was difficult to communicate directly, both for social and technical reasons.
The role of climatic factors in influencing the introduction or reappearance and transmission of infectious diseases in free geographical areas can be multiple, but extremely complex. In this regard, the use of predictive geo-informatics, the systems of remote detection of climate models associated with stratification of risks, are exploited to optimize the planning and development of models of transmission of vector diseases.
The variety of phenomena to which the datasets can refer, allow to aggregate and to relate a large set of data to support vector control; they are also used to develop models of transmission that permits to detect early on the possible incursions of diseases or topredict their reappearance.
An example of this is the application of spatial analysis techniques as predictive tools for the evaluation of West Nile Disease transmission, for which it is of fundamental importance to identify early the areas and periods with the greatest risk of disease transmission. This type of model uses environmental, climatic and territorial variables to identify habitats suitable for the spread of the virus and to produce monthly maps of vector spread according to the seasonality.
Social media is also an effective alternative source of large-scale, real-time geospatial data and has been used as a case study for the spread of the Chikungunya virus in Europe. In fact, the analysis of Twitter's geocoded Big data associated with those related to air traffic from international areas where the disease is endemic, has provided useful information to identify the short-range dispersion of the virus from the outbreaks of origin. Moreover, through the calculation of the estimate of the seasonal vector capacity of Aedes Albopictus, it was understood that the growth of the population of infected vectors was also influenced by local seasonal climatic variables in which temperatures higher than the average might determined a significant increase in the vector capacity and therefore in the transmission of the disease (Figure 2).
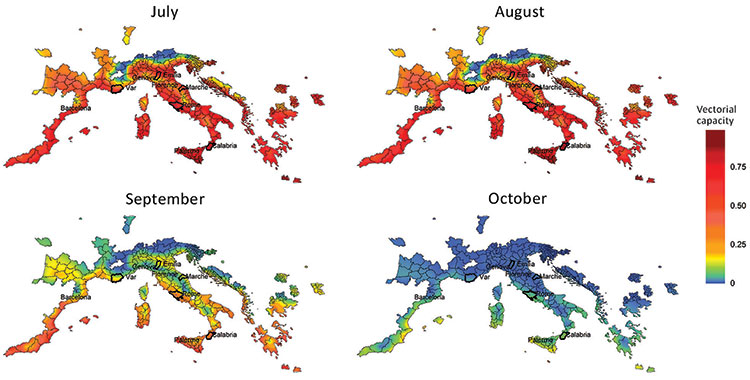
Figure 2. Vectorial capacity estimates based on average temperature conditions in Europe (Italy and France) with stable populations of Aedes albopictus mosquitoes around chikungunya outbreak zones. Heavy outlines indicate the outbreak areas. (Rocklöv et al., 2019)
However, the results of local spread mobility models obtained need epidemiological confirmation by phylogenetic analysis; although they have good sensitivity in identifying areas at risk of virus spread based on mobility and climate data, they need to improve in specificity, including local contextual factors such as land use and vector activity. Wikipedia and Google trends were also proposed as data resources for disease surveillance and epidemic detection - and in the specific case of Chikungunya virus - but were found to be of little use for an early response to the disease, as they seemed to indicate mainly and exclusively a public awareness of the presence of the outbreaks and not their actual location.
Big data represent a new frontier in detecting interactions between numerous variables represented by human, environmental and animal systems. However, their full use is still limited by the difficulties that emerge in their organization, because flows without a strong structure often not supported by the adequate technical skills needed to aggregate them, but it is also limited in their use by the management of sensitive data.
However, due to their low cost of generation and high usability, when used consciously to prevent and control emerging and re-emerging diseases, they allow rapid planning in the preparation and response to outbreaks, and moreover, it helps to identify areas at risk where control measures will have to be implemented in a timely manner. This strategy makes it possible to achieve the objectives of the One Health approach, which aims to provide rapid responses at minimal cost in the prevention and monitoring of zoonoses.
References
- Asokan G.V., Asokan V. (2015). Leveraging “Big Data” to enhance the effectiveness of One Health in an era of health informatics Journal Epidemiology Global Health, pp.311-314
- Conte A., Candeloro L., Ippoliti C., Monaco F., De Massis F., Bruno R., Di Sabatino D., Danzetta M.L., Benjelloun A., Belkadi B., El Harrak M., Declich S., Rizzo C., Hammami S., Ben Hassine T., Calistri P., Savini G (2015). Spatio-Temporal Identification of Areas Suitable for West Nile Disease in the Mediterranean Basin and Central Europe. PLoS One, 2015 Dec 30; 10(12)
- Rocklöv J., Tozan Y., Ramadona A., Sewe M.O., Sudre B., Garrido J., de Saint Lary C.B., Lohr W., Semenza J.C. (2019). Using Big Data to Monitor the Introduction and Spread of Chikungunya, Europe, 2017. Emerging Infectious Disease, 2019 Jun; 25(6):1041-1049
- Kilpatrick A.M., Randolph S.E. (2012). Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. Dec 1; 380
- Hufnagel L., Brockmann D., Geisel T. (2004). Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA 101:15124–29
- Global vector control response
- European network for sharing data on the geographic distribution of arthropod vectors, transmitting human and animal disease agents (VectorNet).
Alessandra Di Giuseppe
Centro Operativo Veterinario per l'Epidemiologia, Programmazione,
Informazione e Analisi del Rischio
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale"