The emerging worldwide warning of antifungal resistance
Fungi are key components in global biogeochemical cycles, play important roles in manufacturing industries and biomedical research, and influence humans through their impact on global health, agriculture, and biodiversity. Fungi have been isolated from almost every environmental niche across the planet, including from air, soil, freshwater, and the oceans. Traditionally, fungi are identified by their morphological characteristics but, with the progress of molecular biology and sequencing techniques, identification and characterization of fungal species have become increasingly dependent on DNA polymorphisms and mitochondrial genome.
Fungal genomes tend to be highly plastic and evolutionary with wide genetic diversity among natural isolates, which is one of the main reasons why antifungal resistance can evolve so rapidly in these microorganisms (Shang Sun et al., 2022).
While the vast majority of fungi are non-pathogenic, a limited number can cause serious diseases in plants and animals (including humans), with potential devastating effects on public health, agriculture, biodiversity and related socio-economic consequences. Indeed, fungal infections, named mycoses, are increasingly becoming a global health problem that is associated with high morbidity and mortality rates. Fungi could be responsible for superficial mycoses that generally require a simple pharmaceutical treatment but also for invasive mycoses (Braun et al, 2012); due to this factor, severity ranges from asymptomatic or mild mucocutaneous infections to potentially life-threatening systemic infections. Mortality associated with fungal disease has been estimated to be approximately 1.7 million deaths per year, similar to that of tuberculosis and three-fold more than malaria (Bongomin et al., 2017).
The rising incidence of mycosis in the last decade is related to fungal pathogenic microorganisms that are increasingly resistant to antifungal treatments. Severe fungal infections mainly occur in immunosuppressed individuals, such as patients affected by Human Immunodeficiency Virus (HIV), individuals with endocrine-metabolic disorders, or those undergoing antineoplastic chemotherapy or immunosuppressive therapy after organ transplantation (Vitiello et al., 2023).
In table 1, are reported data on the annual incidence and the consequent global impact of the main fungal diseases.
Fungi can survive on surfaces for weeks and some of the disinfectants commonly used in hospitals often are not effective. Moreover, the ease spread of microorganism due to global trade, high frequency of travel and climate change makes fungal infections difficult to control.
Table 1. Annual incidence and global prevalence of the main mycosis
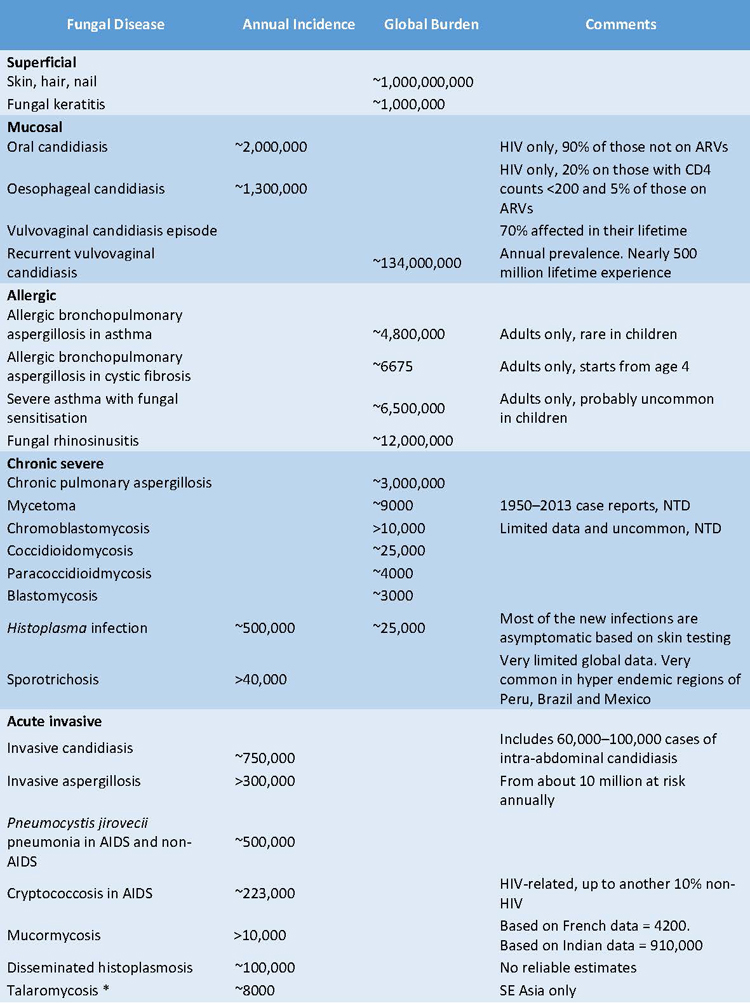
ART: Antiretrovira therapy adherence NTD = WHO-accepted Neglected Tropical Disease
Candida albicans, Candida glabrata, and Aspergillus spp. are among the fungal pathogens most responsible for severe systemic mycoses. In particular, the Aspergillus species are very persistent in the hospital environment causing a wide range of infections, including life-threatening systemic ones, especially in patients with severe immune system impairment (Vitiello et al., 2023). Recently, the World Health Organization (WHO) developed the first fungal priority pathogens list (FPPL) that includes the 19 fungi representing the greatest threat to public health, divided into three categories: critical, high and medium priority (WHO, 2022).
- The critical priority group includes four pathogens: Cryptococcus neoformans, Candida auris, Candida albicans and Aspergillus fumigatus.
- The high priority group includes seven pathogens: Nakaseomyces glabrata (Candida glabrata), Histoplasma spp., eumycetoma causative agents, Mucorales, Fusarium spp., Candida tropicalis and Candida parapsilosis.
- The medium priority group includes eight pathogens and some of these are confined to certain geographical areas and therefore are not considered a priority globally. However, in the areas where these pathogens are endemic, they are associated with a significant burden of mycosis. An example is Paracoccidioides spp, a pathogenic dimorphic fungi endemic to Central and South America that live in the soil and determines a mortality ranges from 3% to 23%.
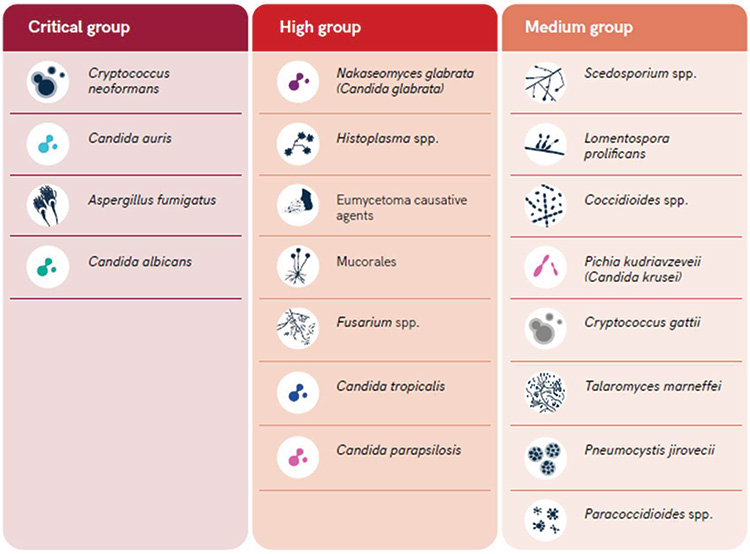
Figure 1. WHO fungal priority pathogens list (2022)
The example of Candida auris well describes the emerging problems related to antifungal resistance. C. auris seems to be significantly less susceptible to several standard healthcare disinfectants and can be easily transmitted from patient to patient, which is an unusual characteristic for fungal pathogens. The pharmacological options for antifungal treatment are currently limited to three distinct pharmacological classes: azoles, echinocandins and polyenes. It is alarming to note that in 1% of isolates, C. auris is resistant to all three classes of antimycotics, about 90% of all isolates are resistant to at least one class of antifungal drugs and about 30% to at least two classes. Unfortunately, multidrug resistance is also gaining ground in other fungi, mostly in the Candida species (the most widespread fungal pathogens) such as in the Candida glabrata, where the cross-resistance between azoles and echinocandins is increasingly recognized (Kainz et al., 2020).
The sensitivity of the main pathogenic fungi to certain antifungals treatments of the three most important classes of antifungals, are detailed in Table 2 (Vitiello et al., 2023).
Table 2. Sensitivity to the main antifungal treatments used in the clinical practice against the most frequent
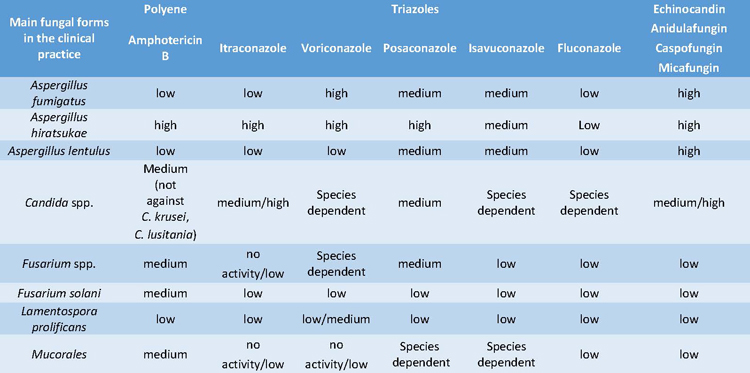
Azoles are the most clinically relevant pharmacological subgroup, since most azoles show comparably high efficacy, low toxicity, immunomodulatory capacity and the possibility of oral application (Campoy et al., 2017). These advantages have encouraged their long-term therapeutic application and prophylactic use in high-risk patients, which has led to an increase in less sensitive strains.In 2018, the WHO launched a pilot Candida surveillance program to gather retrospective data on antifungal resistance for invasive isolates of Candida; this was recently formally included in the Global Antimicrobial Resistance Surveillance System (GLASS) program. In addition to these broader and more systematic surveillance programs, nationwide surveillance data are available for Candida spp. from several countries such as Australia, Scotland, Finland, Iceland, Norway, Sweden, the United Kingdom and Denmark (Astvad et al., 2018). Nevertheless, surveillance of other fungal species is rare and most published data is restricted to azole-resistant A. fumigatus.
There is an eco-evolution link between environmental and clinical resistance in fungal infections and treatments that allows to underline how this emerging threat requires a One Health approach. Humans are exposed daily to opportunistic pathogenic fungi commonly found within close living environments, and the intimate relationship between environmental populations of fungi and ensuing exposures to antifungals, means that emerging environmental resistance is likely to affect the clinical management of fungal infections.
In the agricultural setting, phytopathogenic fungi develop continuous resistance to the array of fungicides deployed against them. Fungicides used in agriculture have broad spectrum activity across the fungal kingdom and so resistance arises not only in the crop pathogens per se but also in other environmental fungi that include potential human fungal pathogens.Another important aspect is the higher average temperatures expected under climate change scenarios that may affect the emergence of antifungal resistance. Fungi respond to temperature by regulating cell membrane lipid composition, which in turn alters indirectly antifungal resistance, as demonstrated by the high frequency of azole-resistance by A. fumigatus in environments with higher average temperatures such as tropical countries (Fisher et al., 2022).
In Table 2, it is reported the annual prevalence of invasive aspergillosis in 37 countries accounting for a global population higher than 2,000 million people, nearly the 29% of the world population. The maximum number of invasive aspergillosis cases have been observed in Vietnam with 14,523 yearly infected people (Bongomin et al., 2017).
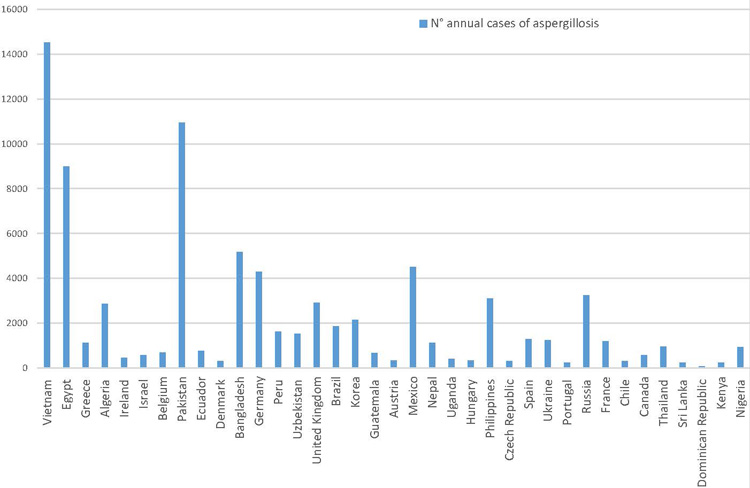
Figure 2. Published country estimates of invasive aspergillosis
The threat imposed by fungal infections will continue to increase worldwide with many obstacles that need to be overcome with rapid and innovative action at different levels. Firstly, the search for therapeutic options needs to be intensified: besides, searching within the classical antifungal drug pipeline, novel therapeutic strategies might be found. Numerous candidate fungal vaccines have been studied in the preclinical setting, but only the C. albicans recombinant Als3 protein vaccine has shown promising results in phase II clinical trials (Edwards et al., 2018).
Secondly, antifungal susceptibility testing needs to be further standardized, since the current lack of unified protocols causes discrepancies between laboratories and difficulties in the interpretation of data obtained, making it hard to find the optimal treatment option for a patient and increasing antifungal resistance. Thirdly, the awareness of fungal infections at the social and governmental levels must raise because, despite the huge number of lethal infections, their impact remains rather underestimated (Kainz et al., 2020).
The development of rapid genomic analysis has been the key to understanding the international local-scale transmission of C. auris, including the emergence of multidrug-resistant variants.
The implementation of whole-genome sequencing (WGS) is promising and it is a useful tool for examining the biological basis of gene mutations. However, in contrast to antibacterial resistance, a standardized WGS typing method is not widely used for fungi because of their larger genome sizes, frequent sexual recombination and lack of standardized bioinformatic pipelines (Fisher et al., 2022).References
- Astvad KMT, Johansen HK, Røder BL, et al. Update from a 12-year nationwide fungemia surveillance: Increasing intrinsic and acquired resistance causes concern | journal of clinical microbiology. Journal of Clinical Microbiology. 2018.
- Edwards John E. Jr, Schwartz Michael M, Schmidt Clint S, et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal Candidiasis—A phase 2 randomized, double-blind, placebo-controlled trial. Clinical Infectious Diseases. 2018;66(12):1928-1936.
- Duong Tra-My N, Le Thanh-Van, Tran Khanh-Linh, et al. Azole-resistant aspergillus fumigatus is highly prevalent in the environment of vietnam, with marked variability by land use type. Environmental Microbiology. 2021;23(12):7632-7642
- Perlin David S, Rautemaa-Richardson Riina, Alastruey-Izquierdo Ana. The global problem of antifungal resistance: Prevalence, mechanisms, and management. THE LANCET - Infectious diseasis. 2017;17(12):e383-e392. Ultima consultazione 24 novembre 2023. doi: https://doi.org/10.1016/S1473-3099(17)30316-X
- Campoy Sonia, Adrio Jose L. Antifungals. Science Direct. 2017. Ultima consultazione 24 novembre 2023
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: Human fungal infections. Sci Transl Med. 2012;4(165):165rv13. Ultima consultazione 24 novembre 2023
- WHO fungal priority pathogens list to guide research, development and public health
action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. In: 2022
- Castanheira M. Fungemia surveillance in Denmark demonstrates emergence of non-albicans candida species and higher antifungal usage and resistance rates than in other nations. Journal of clinical microbiology. 2018;56(4). doi: 10.1128/JCM.01907-17
- Sung Shen, HoyMichael J, Heitman Joseph. Fungal pathogens – Science Direct. 2020. Ultima consultazione 7 novembre 2023
- Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D. Fungal infections in humans: The silent crisis. Microb Cell. 2020;7(6):143-145. Ultima consultazione 17 ottobre 2023
- Vitiello A, Ferrara F, Boccellino M, et al. Antifungal drug resistance: An emergent health threat. Biomedicines. 2023;11(4):1063. doi: 10.3390/biomedicines11041063
- Bongomin F, Gago S, Oladele R, Denning DW. Global and multi-national prevalence of fungal Diseases—Estimate precision. JoF. 2017;3(4). doi: 10.3390/jof3040057
-
Fisher Matthew C, Alastruey-Izquierdo Ana, Berman Judith, et al. Tackling the emerging threat of
antifungal resistance to human health. Nature Reviews | MICROBIOlOGY. 2022;20. doi: https://doi.org/10.1038/ s41579-022-00720-1
Capone M. R.
Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale”
Centro Operativo Veterinario per l'Epidemiologia, Programmazione, Informazione e Analisi del Rischio (COVEPI)
Corresponding author: m.capone@izs.it