Leptospirosis: let’s not forget it!
Overview of leptospirosis
Leptospirosis is a worldwide zoonotic disease caused by Gram negative bacteria of the genus Leptospira spp. The taxonomy of Leptospira is complex and it is based on two different classification criteria. Serologically, there are more than 300 leptospiral serovars recognised, and these are grouped into 30 serogroups. According to the genetic classification, instead, 66 genospecies, classified across four subclades, are currently known. Pathogenic strains of the genus Leptospira belong primarily in the P1 subclade (WOAH, 2021).
In western countries leptospirosis is considered a neglected zoonosis with an underestimated prevalence. Despite this, it represents a serious threat to public health, especially in developing countries, where the highest prevalence rates are reported. It is estimated that more than one million human cases occur worldwide each year, resulting in about 59,000 deaths (CDC, 2021). Occupational exposure, international travel and outdoor recreational activities, such as swimming, rafting, fishing, or gardening, are associated with a higher risk of infection, due to the intense contact with animals, water or soil (Schuller et al., 2015).

Figure 1. Transmission cycle of pathogenic Leptospira spp. (created with BioRender.com)
Leptospirosis can affect several wild and domestic mammalian species, which act either as accidental hosts or maintenance hosts for a specific serovar (Figure 1). Maintenance hosts, such as rats for the serogroup Icterohaemorrhagiae, seldom develop clinical signs of the disease, but they represent a natural source of infection as they harbor leptospires in the kidney and they persistently excrete them via urine, contaminating the environment. Accidental hosts can then become infected through direct contact of mucous membranes or broken skin with infected urine or contaminated soil and surface water (Schuller et al., 2015).
The survival of pathogenic leptospires in the environment is promoted by the presence of stagnant water sources, mud, and mild temperatures, whereas the bacteria are rapidly inactivated by direct light, salt water, low temperatures and lack of humidity. In fact, despite its worldwide distribution, leptospirosis is more commonly reported in the tropics (Bharti et al., 2003). However, global climate change and the increased frequency of extreme weather events such as floods or heavy rainfalls might result in an upsurge in the disease incidence also in temperate regions (Lau et al., 2010).
Until the early Sixties, in Italy, particularly in northern regions, leptospirosis was a common occupational infection, especially in rice-fields workers. With the subsequent gradual mechanization of rice cultivation and the application of prophylaxis measures for the subjects at risk, the disease became sporadic in humans (Ciceroni et al., 2000), whereas nowadays it is still diagnosed in livestock and companion animals, mainly in dogs.
Canine leptospirosis
Dogs are highly susceptible to leptospiral infection, and they can develop severe forms of the disease, such as the uremic syndrome, the icterohaemorrhagic syndrome, and the pulmonary haemorrhagic syndrome. According to the serovar involved, infection with pathogenic leptospires can lead to a wide range of clinical signs from subclinical to hyperacute, primarily related to renal and hepatic disease.
Diagnosis of canine leptospirosis is complex, and it should be based on the evaluation of anamnestic and clinical data, as well as of laboratory test results. In case of suspected infection, it is recommended to follow some specific diagnostic protocols (IZSVe, 2020). These include serological tests, such as the microscopic agglutination test (MAT), which is the international gold standard method, and molecular biology tests (PCR or Real-Time PCR), ideally combined with blood and urine analyses.
Urinary shedding is often intermittent in dogs, even in the most severe cases. However, dogs play an important role in the epidemiology of leptospirosis since they serve as an indicator of the serovars circulating in the environment and act as a sentinel species for the risk of exposure to humans. The main serogroups associated with canine leptospirosis in Europe are Icterohaemorrhagiae, Grippotyphosa, Australis, Sejroe, and Canicola (Balboni et al., 2021). Dogs can represent reservoir hosts for Canicola and Australis, whereas rats and hedgehogs are considered the most important maintenance hosts for the other serogroups. Consequently, the epidemiology of canine leptospirosis may vary by geographic area and change over time in relation to the spread of maintenance hosts and to the use of vaccines (Suepaul et al., 2010; Balboni et al., 2021). Vaccination is an effective way to prevent clinical manifestations of the disease and to reduce bacterial shedding. In Europe, currently commercialized vaccines are either bivalent (protection against serogroups Canicola and Icterohaemorrhagiae), trivalent (protection against Canicola, Icterohaemorrhagiae, and Grippotyphosa), or tetravalent (protection against serogroups Canicola, Icterohaemorrhagiae, Grippotyphosa, and Australis).
The current epidemiological situation in North-East Italy: updates from the RC 05/17 IZSVe project
Recently the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe) has carried out a project aimed at studying thoroughly the epidemiological knowledge on canine Leptospirosis in the Triveneto area. In particular, the research was addressed at:
- Identifying leptospiral serovars circulating in the area, with enhancement of the available molecular typing database
- assessing the adequacy of vaccines currently available for dogs
- evaluating the climatic-environmental factors which influence the risk of infection
- defining the risk of infection to humans in categories of “exposed” (veterinarians) and “non-exposed” (blood donors) subjects.
The results provided by this study were integrated with historical data collected by the National Reference Centre for Animal Leptospirosis (CRNL), and with the results obtained in a previous project conducted by the Istituto Zooprofilattico Sperimentale delle Venezie (RC IZSVE 16/12).
The project involved 126 private veterinary facilities which provided data and samples related to 617 cases of suspected clinical leptospirosis between January 2018 and August 2021. In order to confirm the diagnosis of leptospirosis and to identify the genospecies and the serovars involved, samples were subjected to serological testing (MAT), microbiological analyses (isolation by EMJH medium), and biomolecular analyses (Real-Time PCR, MLST, MLVA). Out of 617 suspected cases 211 were confirmed cases on the basis of positive Real-Time PCR and/or isolation and/or positive MAT results with antibody titer > 1:400 for at least one serovar included in the antigen panel. Laboratory analyses confirmed what previously observed in the RC IZSVE 16/12 project. Six distinct Sequence Types (ST), associated with 3 genospecies, were found in confirmed cases. Leptospira strains belonging to serogroup Icterohaemorrhagiae (ST17) were the most detected ones, followed in descending order of prevalence by strains of serogroups Australis (ST198 and ST24), Pomona (ST117 and ST289), and Sejroe (ST155). Table 1 provides results of the genotyping analysis carried out by the CRNL and the IZSVe between 2013 and 2020, whereas Figure 2 shows locations and amounts of the genotyped strains, assessed by Multilocus Sequence Typing (MLST).
Table 1. Sequence Types (STs) detected by MLST in samples collected by the CRNL and the IZSVe between 2013 and 2020
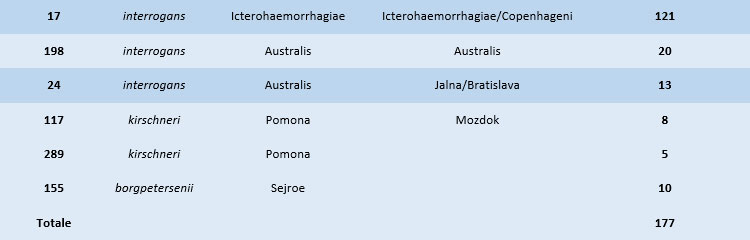

Figure 2. Geographical distribution of Sequence Types (STs) detected through genotyping analysis (MLST). The map was generated combining data collected by the CRNL and the IZSVe between 2013 and 2020
To evaluate the epidemiological role of wildlife in the spread of Leptospira strains relevant to dogs and humans, samples from wild and synanthropic species were also investigated. With exception of ST289, all of the strains detected in dogs were also found in other host species. In particular, ST17, which occurred very frequently in dogs, was also found in 21 rats, 2 mice and one fox. Furthermore, the genotyping analysis highlighted STs which had not been detected in dogs: ST146 (L. borgpetersenii Javanica) was found in two foxes and one hedgehog, ST149 (L. borgpetersenii Ballum) was identified in one hedgehog, and a new ST (not included in the international database) was found in a vole. Genotypes detected in dogs and in the wild species described were compared using a minimum spanning tree analysis (Figure 3).
The climatic-environmental analysis did not identify the presence of a particular spatial trend in the confirmation of cases, nor the influence of seasonality and year on the distribution of the cases. At the same time, the analysis of data collected through an epidemiological questionnaire related to clinical cases recorded in Veneto highlighted how recent exposure to freshwater sources or rodents can increase the probability of case confirmation by 2.36 times, whereas vaccination (which allows to reduce the probability of infection by 50%) and frequentation of urban environments were protective factors.

Figure 3. Minimum Spanning Tree based on Sequence Types (STs). Each circle represents one specific ST, and the number of sectors corresponds to the number of samples having that ST
The serological investigation aimed at assessing the risk of infection in categories of professionally exposed and non-exposed subjects, conducted on 211 people, did not show any significant difference in seroprevalence between the two groups (Mazzotta et al., 2022). This may be due both to the awareness of the risk and the use of adequate personal protective equipment in veterinary practice, as well as to the relatively low excretion of Leptospira in symptomatic dogs, which can be considered as an environmental sentinel for Leptospira presence rather than a vehicle of transmission.
The results of the present study showed epidemiological relationships between dogs and wild species, as well as a high prevalence of serogroup Icterohaemorrhagiae in the clinical cases investigated. These findings corroborated the hypothesis that Leptospira infection in dogs is the consequence of environmental contamination spread by rodents, which act as main reservoir hosts of Icterohaemorrhagiae and Copenhageni serovars. The role of hedgehogs, maintenance hosts for serogroup Australis, is also worthy of attention. Furthermore, the study highlighted the usefulness of introducing vaccination coverage also for serogroups Pomona and Sejroe, currently circulating in Italy yet not included in any commercial vaccine.
Finally, it is important to encourage the use of genotyping methods for the identification of circulating Leptospira strains in order to deepen the knowledge on the spread of leptospirosis in Italy. Despite leptospirosis is a notifiable disease, official reports are scarce because diagnosis is often made by private veterinarians. Entrusting the Istituti Zooprofilattici Sperimentali with the task of performing diagnostic testing on behalf of private veterinary practitioners would allow them to act as an epidemiological observatory on leptospirosis, adopting a One Health approach and ensuring positive outcomes for human health as well.
References
- Balboni, A., Mazzotta, E., Boniotti, M. B., Bertasio, C., Bellinati, L., Lucchese, L., Battilani, M., Ceglie, L., Marchione, S., Esposito, G., & Natale, A. (2022). Outbreak of Leptospira borgpetersenii Serogroup Sejroe Infection in Kennel: The Role of Dogs as Sentinel in Specific Environments. International Journal of Environmental Research and Public Health, 19(7), 1–12
- Bertasio, C., Boniotti, M. B., Lucchese, L., Ceglie, L., Bellinati, L., Mazzucato, M., Furlanello, T., D’incau, M., Natale, A. (2020). Detection of new leptospira genotypes infecting symptomatic dogs: Is a new vaccine formulation needed? Pathogens, 9(6), 1–20
- Bharti, A. R., Nally, J. E., Ricaldi, J. N., Matthias, M. A., Diaz, M. M., Lovett, M. A., Levett, P. N., Gilman, R. H., Willig, M. R., Gotuzzo, E., Vinetz, J. M., & Peru-United States Leptospirosis Consortium (2003). Leptospirosis: a zoonotic disease of global importance. The Lancet. Infectious diseases, 3(12), 757–771
- Centers for Disease Control and Prevention (CDC) (2021). Healthcare Workers - Technical Information for Leptospirosis. U.S. Department of Health & Human Services
- Ciceroni, L., Stepan, E., Pinto, A., Pizzocaro, P., Dettori, G., Franzin, L., Lupidi, R., Mansueto, S., Manera, A., Ioli, A., Marcuccio, L., Grillo, R., Ciarrocchi, S., & Cinco, M. (2000). Epidemiological trend of human leptospirosis in Italy between 1994 and 1996. European Journal of Epidemiology, 16(1), 79–86
- Lau, C. L., Smythe, L. D., Craig, S. B., & Weinstein, P. (2010). Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Transactions of the Royal Society of Tropical Medicine and Hygiene, 104(10), 631–638
- Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe) (2020). Percorsi diagnostici negli animali da compagnia - Leptospirosi canina. IZSVe Pets
- Mazzotta, E., Lucchese, L., Salata, C., Furlanello, T., Baroni, E., Zotti, A., Venturi, G., Fincato, A., Marchione, S., Capello, K., & Natale, A. (2022). Are Small Animal Practitioners Occupationally Exposed to Leptospirosis? Results of a Serological Survey. International Journal of Environmental Research and Public Health, 19(3)
- Schuller, S., Francey, T., Hartmann, K., Hugonnard, M., Kohn, B., Nally, J. E., & Sykes, J. (2015). European consensus statement on leptospirosis in dogs and cats. Journal of Small Animal Practice, 56(3), 159–179
- Suepaul, S. M., Carrington, C. V. F., Campbell, M., Borde, G., & Adesiyun, A. A. (2010). Serovars of Leptospira isolated from dogs and rodents. Epidemiology and Infection, 138(7), 1059–1070
- World Organisation for Animal Health (WOAH) (2021). Leptospirosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022. Chapter 3.1.12. WOAH, Paris, France.
Tassinato C.1, Mazzotta E.1, Lucchese L.1, Boniotti M.B.2, Bertasio C.2, D’Incau M.2, Ceglie L.1, Bellinati L.1, Mazzucato M.1,
Natale A.1,*
1Istituto Zooprofilattico Sperimentale delle Venezie
2Centro di Referenza Nazionale per la Leptospirosi, c/o Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna “Bruno Ubertini”
*Referente: anatale@izsvenezie.it