Rift Valley fever: a new threat for Europe just around the corner
Rift Valley fever (RVF) virus is a single negative-strand RNA virus, belonging to the order Bunyavirales, the family Phenuiviridae and the genus Phlebovirus. RVF is a mosquito-borne viral disease affecting both domestic and wild ruminants, especially sheep, cattle and goats as well as humans (OIE, 2019) and it is transmitted primarily by Culex spp. and Aedes spp. mosquitoes (Balkhy et al., 2003).
The RVF is characterised by the so called “abortions storms”, involving almost 100% of susceptible pregnant ruminants and by high mortality in young animals (Anyangu et al., 2010). The abortion products represent the main infection source for humans (Zeller et al., 1997).
The susceptibility of different species and breeds to RVF may vary considerably and mortality rate depends upon the species and age of the animal, with sheep and goats generally being more susceptible to death than cattle. Camels usually have an unapparent infection, but sudden mortality, neonatal mortality and abortion rates can be as high as in cattle (OIE, 2016).
The infection in humans can determine different clinical forms of disease from moderate to severe and, in its most severe forms, the illness can progress to haemorrhagic fever, encephalitis, or ocular disease with fatal prognosis (Ikegami et al., 2011).
Although most of the sub-Saharan countries are considered to be part of the endemic area for RVF, the disease has several times proven to be able to spread to new territories, such as in Saudi Arabia and Yemen in 2000, or to regularly occur in Egypt at higher latitudes along the Nile river.
In January 2020, Libya officially notified, for the first time, two RVF outbreaks to the OIE (WAHIS, 2020; Nielsen et al., 2020) in the South East. The first two outbreaks were followed by the notification of other six outbreaks. Five outbreaks are concentrated in the south-eastern district of Alkufrah, whereas the other three have been identified in the central and south-western part of Libya (Figure 1).
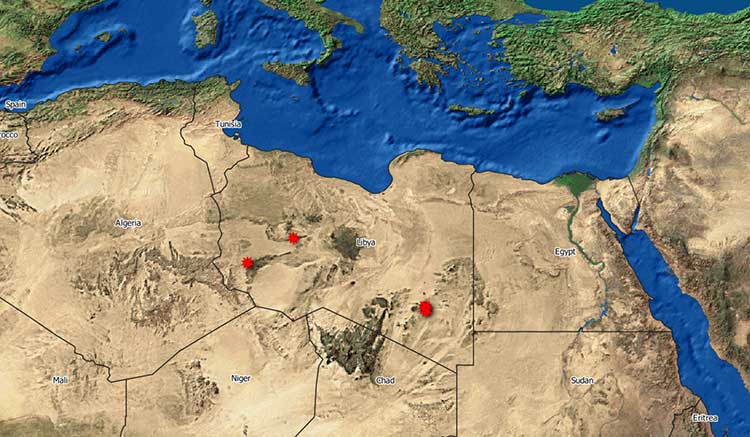
Figure 1. Geographical distribution of RVF outbreaks in Libya (Source:WAHID-OIE 2020)
Regarding these outbreaks, the lack of major epidemiological information, as the origin of animals, hindered any epidemiological evaluation and assumption about the origin of these two outbreaks, altough it is noteworthy that the beginning of the Libyan’s outbreak was reported as 12 December 2019, more or less the same period of a large RVF epidemic notified in Sudan (Nielsen et al., 2020).
The instability in Libya and increasing insecurity in the Sahel and Sahara regions could have been potential factors for altering the main livestock trade routes (Nielsen et al., 2020; EFSA, 2015). Unofficial animal movements between Sahel and Maghreb countries are well documented like dromedary camels probably arriving from Mauritania were found RVF positive in southern Morocco (El-Harrak et al., 2011; Nielsen et al., 2020) and small ruminants serologically positive for RVF in the Sahrawi territories where animals are traded between Mali and Mauritania towards Algeria (Di Nardo et al., 2014; Nielsen et al., 2020). Animals originating from Chad and Sudan have been found in Libya, as well as sheep from Mali in the centre of Tunisia (Bouguedour et al., 2016; Nielsen et al., 2020). In addition, in a recently published paper (Selmi et al., 2020) around 34% of dromedaries tested in Tunisia showed antibodies against RVF virus.
In this context, the presence of the infection in Libya should raise the awareness of Italy, given its proximity to the Libyan coasts and considering that infected vectors could be transported over the sea by winds and introduced to the southernmost territories of Italy. It must be also taken into account that the social detriment situation due to the war in Libya and the contemporaneous presence of SARS-CoV-2 infection could substantially delay the recognition of RVF spread in human and animal populations in this country.
For all these reasons, the European Food Safety Authority (EFSA) recommended in a recent Scientific Opinion (Nielsen et al., 2020) that: “Considering the possible future source of risk represented by the spread of infection into new areas closer to the EU borders, it is of paramount importance for the EU to establish and maintain a close collaboration with North African and Middle Eastern countries in the surveillance of possible introduction of RVF from currently infected areas, as well as to carefully monitor the evolution of the epidemics in African countries”.
A key country in the Maghreb, for its strategic position and closeness to Libya, is certainly Tunisia. It is of paramount importance to increase all collaboration efforts with Tunisia for supporting this country in the surveillance of possible entrance of RVF into its territory, and in case, for the prompt reaction to control the spread of the disease. Any difficulty or delay in reacting by Tunisia to a possible introduction of RVF will be paid not only by Tunisia, but also by the closest countries, such as Italy.
Regarding surveillance in virus-free areas, like Tunisia or Italy, the main objective would be to have an effective early warning system, be able to detect and confirm the presence of the infection, in case of its introduction. For this purpose, the use of a dense sentinel herd network would be cost prohibitive unless strictly focused on ecologically defined risky areas (Chevalier, 2013).
The introduction of RVF into a fully susceptible ruminant population, like those in Tunisia or Italy, will determine a conspicuous number of clinical cases, with high mortality in young animals and abortion storms. For this reason syndromic or passive surveillance would be the best surveillance approach in free countries. Syndromic surveillance relies on the early detection of abnormal clusters of illness indicators rather than clinical signs, and thus reduces the time-lag between the onset of the outbreak and the diagnosis (Chevalier, 2013). This methodology may be a useful alternative in the case of RVF, which may provoke non-specific signs, in either animals or humans (Chevalier, 2013).
A re-enforced surveillance on abortions, stillbirths and neonatal mortality, therefore, should be applied during summer and autumn in the areas at major risk of introduction. In case of abnormal abortion or mortality rates, all aborted foetuses and dead animals should be investigated also the presence of RVF virus during the vector season.
The veterinary services, including the veterinary diagnostic system, should be well prepared and trained to react immediately in case of suspect of the disease and procedures for the possible use of vaccines should be already evaluated and ready to be activated.
RVF is just around the corner and the epidemiological context of RVF in North Africa is indicating that it is time for Italy to re-inforce its entire preventive measures to be ready in case the most unfavourable scenario will occur.
References
- Anyangu A.S., Gould L.H., Sharif S.K., Nguku P.M., Omolo J.O., Mutonga D., Rao C.Y., Lederman E.R., Schnabel D., Paweska J.T., Katz M., Hightower A., Njenga M.K., Feikin D.R. & Breiman R.F. 2010. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 83(2 Suppl):14-21
- Balkhy H.H. & Memish Z.A. 2003. Rift Valley Fever: An uninvited zoonosis in the Arabian Peninsula. Int J Antimicrob Agents. 21,153–157
- Bouguedour R. & Ripani A. 2016. Review of the foot and mouth disease situation in North Africa and the risk of introducing the disease into Europe. Rev Sci Tech. 35, 757–768
- Chevalier V., 2013. Relevance of Rift Valley fever to public health in the European Union. Clin Microbiol Infec, Volume 19 Number 8
- Di Nardo A., Rossi D., Saleh S.M.L., Lejlifa S.M., Hamdi S.J., Di Gennaro A., Savini G. & Thrusfield M.V., 2014. Evidence of Rift Valley fever seroprevalence in the Sahrawi semi-nomadic pastoralist system, Western Sahara. BMC Vet Res. 10, 92
- El-Harrak M., Martín-Folgar R., Llorente F., Fernández-Pacheco P., Brun A., Figuerola J. & Jiménez-Clavero M. 2011. Rift Valley and West Nile Virus Antibodies in Camels, North Africa. Emerg. Infect. Dis. 17, 2372–2374
- European Food Safety Authority (EFSA) 2005, AHAW Panel Members, Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to ‘‘The risk of a Rift Valley fever incursion and its persistence within the Community’’,11 October 2005, pp. 1–130
- Ikegami T. & Makino S. 2011. The pathogenesis of Rift Valley Fever. Viruses 3, 493–519
- Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin-Bastuji B, Rojas JLG, Schmidt CG, Michel V, Chueca MAM, Roberts HC, Sihvonen LH, Stahl K, Calvo AV, Viltrop A, Winckler C, Bett B, Cetre-Sossah C, Chevalier V, Devos C, Gubbins S, Monaco F, Sotiria-Eleni A, Broglia A, Abrahantes JC, Dhollander S, Van Der Stede Y and Zancanaro G, 2020. Rift Valley Fever – epidemiological update and risk of introduction into Europe. EFSA Journal 2020;18(3):6041, 72 pp
- Selmi R, Mamlouk A, Ben Said M, Ben Yahia H, Abdelaali H, Ben Chehida F, Daaloul-Jedidi M, Gritli A, Messadi L. First serological evidence of the Rift Valley fever Phlebovirus in Tunisian camels. Acta Trop. 2020 Jul;207:105462. doi: 10.1016/j.actatropica.2020.105462. Epub 2020 Apr 20. PMID: 32325049
- World Organisation for Animal Health (OIE) 2016. Rift Valley fever (infection with Rift Valley fever virus). OIE Terrestrial manual 2016
- World Organisation for Animal Health (OIE) 2019 Technical Card. Rift valley fever
- World Organisation for Animal Health (OIE) WAHIS, 2020
- Zeller H.G., Fontenille D., Traore-Lamizana M., Thiongane Y. & Digoutte J.P. 1997 Enzootic activity of Rift Valley Fever virus in Senegal. Am J Trop Med Hyg. 56, 265–272.
Paolo Calistri
Centro Operativo Veterinario per l'Epidemiologia, Programmazione,
Informazione e Analisi del Rischio
Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale"