West Nile Virus: Twenty Years On
Between 2010 and 2024, at least one autochthonous human case of West Nile fever was reported in 25 European countries, including Italy (1). The progressive increase in confirmed cases, both in humans and in animals, has made this infection an emerging public health issue, highlighting the need for continuous surveillance and targeted prevention measures.
West Nile Virus (WNV) is an RNA, neurotropic, and zoonotic pathogen belonging to the genus Flavivirus, family Flaviviridae. Its transmission cycle primarily involves birds and ornithophilic mosquitoes and occasionally humans and equids, which are considered dead-end accidental hosts. Accidental hosts do not develop a viremia of sufficient intensity to allow the infection of a new vector and are therefore unable to sustain the transmission cycle (2). While a broad range of bird species can be infected, corvids and certain raptor families appear to be particularly susceptible (3), often developing neurological symptoms and experiencing high mortality rates.
In Europe, WNV outbreaks have been predominantly associated with mosquito species of the Culex genus, although other genera and species may also act as vectors (4). Culex mosquitoes typically breed in stagnant water sources such as puddles, irrigation canals, roadside drainage ditches, drinking troughs, and fountains. These mosquitoes are mainly active during dusk and early evening. Humans generally acquire the infection through mosquito bites, although direct transmission can also occur through contaminated blood transfusions, organ or tissue transplants (5).
Multiple genetic lineages of WNV have been identified globally, but most of the severe epidemics have been caused by Lineage 1 and Lineage 2 strains. Phylogenetic studies suggest that the European strains belonging to Lineages 1 and 2 were introduced independently on a limited number of occasions—likely from Africa—followed by local spread and endemic establishment (6).
In humans, approximately 80% of infections are asymptomatic. About 20% of cases develop a flu-like illness characterized by fever, headache, myalgia, and fatigue. In fewer than 1% of cases, the virus can cause severe neuroinvasive disease (West Nile Neuroinvasive Disease – WNND), which may lead to encephalitis, meningitis, or acute flaccid paralysis, particularly in elderly or immunocompromised individuals. To date, no vaccines or specific antiviral treatments are available for humans (7).
In equids, approximately 20% of infections result in clinical manifestations, including fever and neurological symptoms. In unvaccinated horses, the mortality rate can reach up to 50%. The severity of symptoms has been linked to specific viral genotypes, although this association is not always consistent (8).
Geographical Spread and Evolution
Among vector-borne diseases, West Nile Disease (WND) ranks as one of the most widespread globally. West Nile Virus (WNV) was first isolated in 1937 in Uganda from a febrile woman. Since then, it has expanded its geographical range considerably and is now present in Asia, Europe, Australia, and the Americas.
From 1958 onwards, viral circulation began to affect Europe and the Mediterranean basin, with cases reported in France, Portugal, and Cyprus. In 1999, WNV was detected for the first time in the Americas, during a large outbreak in New York City. This event affected wild and zoo birds, horses, and humans, marking the beginning of a rapid westward spread (9). In the following decades, new outbreaks occurred in Bulgaria and Greece (2010), followed by Albania and North Macedonia (2011), and later in Croatia, Serbia, and Kosovo (2012) (10). In 2018, Germany reported its first autochthonous cases in birds (11), followed by the first confirmed human infections in the Netherlands in 2020 (12).
Until 2004, only Lineage 1 strains were circulating in Europe, typically associated with sporadic and milder infections (13). However, Lineage 2—initially restricted to sub-Saharan Africa—emerged in Europe in the early 2000s and has since been responsible for more severe neuroinvasive forms in both humans and horses (14).
Today, West Nile Disease is considered an endemic disease with epidemic potential in Europe, characterized by seasonal transmission cycles that recur annually.
West Nile Virus in Italy: Distribution and Epidemiological Evolution
In Italy, West Nile Virus (WNV) was first reported in 1998 in the Tuscany region, during an outbreak caused by Lineage 1, which resulted in severe clinical symptoms in horses (15).
Following this initial episode, in 2001 the Italian Ministry of Health launched a national veterinary surveillance plan. However, no further cases were detected until 2008. That year, viral circulation was confirmed in the Po River Delta, with WNV detected in mosquitoes, birds, horses, and humans. Since then, the virus has continued to circulate, becoming endemic in several regions of the country, with recurrent seasonal outbreaks (16,17).
Over time, the surveillance system has been progressively updated in response to the evolving epidemiological scenario, with the goal of detecting viral circulation early and rapidly implementing control measures to reduce the risk of human transmission. Since 2008, Italy has experienced sustained WNV circulation, with a significant number of cases reported annually. To date, WNV outbreaks have been recorded every year, supported by genetically distinct viral strains, and cases have been reported in 18 of the 20 Italian Regions (Figure 1a and 1b).
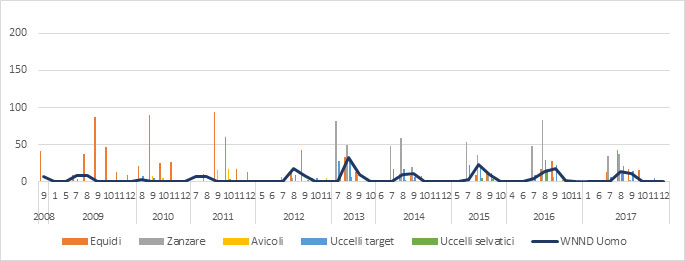
Figure 1a.West Nile Virus Circulation in Italy from 2008 to 2017. Each bar represents the number of WNV-positive cases detected in each animal species under surveillance, broken down by month. The number of human cases of West Nile Neuroinvasive Disease (WNND) reported in the same period is also indicated alongside the veterinary data (Source: Italian National Animal Health Information System-SIMAN)
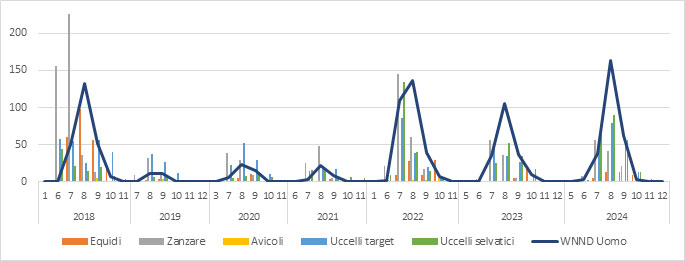
Figure 1b.Circulation of West Nile Virus in Italy from 2018 to 2024. Each bar indicates the number of WNV-positive detections in the various species under veterinary surveillance, broken down by month. The veterinary data are accompanied by the number of reported human cases of West Nile Neuroinvasive Disease (WNND) recorded in the same period (Source: SIMAN)
Circulation of West Nile Virus during the 2024 Epidemic Season

Figure 2.. Geographic distribution of West Nile Virus lineages 1 and 2 in Italy during 2024 (Source: SIMAN)
During the 2024 epidemic season, surveillance activities confirmed, for the first time, the presence of West Nile Virus (WNV) in the Abruzzo region. On June 17, a pool of mosquitoes collected in the province of Chieti tested positive, representing the first evidence of viral circulation in the region since the establishment of the national surveillance system.
In the same week, the virus was also detected in the Veneto region, specifically in the province of Padua. In the following months, several outbreaks were confirmed in the endemic areas of Northern Italy, including Emilia-Romagna, Lombardy, Friuli Venezia Giulia, Piedmont, and Veneto.
Additional positive cases were reported in Central and Southern regions such as Apulia (Puglia), Campania, and again in Abruzzo. In August, the virus was also identified in Tuscany and Sardinia, while by the end of the epidemic season, cases were reported for the first time in the Molise region (Figure 2).
According to official sources (SIMAN and ISS), the West Nile virus detections reported in Italy in 2024 were as follows:
- 66 mosquito pools
- 227 wild birds
- 203 birds belonging to target species (Magpie, Crow, Jay)
- 46 horses showing clinical symptoms (38 outbreaks)
- 460 confirmed human cases, of which 272 presented neuroinvasive forms
(Source: SIMAN)
The maps below (Figure 3a, 3b, 3c) illustrate the geographic expansion of virus circulation (affected areas highlighted in green).
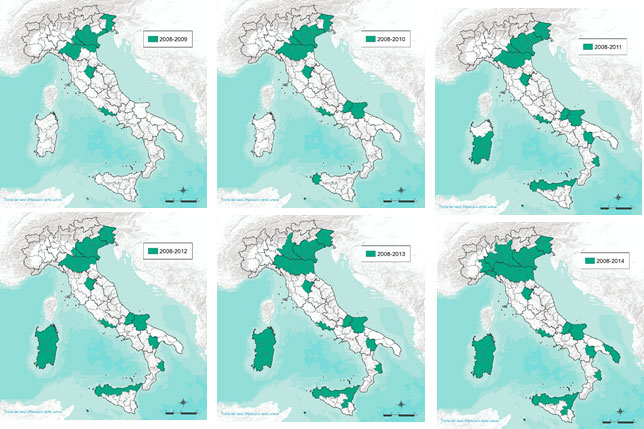
Figure 3a.Distribution of West Nile Virus in Italy (Years 2008–2014)

Figure 3b.Distribution of West Nile Virus in Italy (Years 2015-2020).
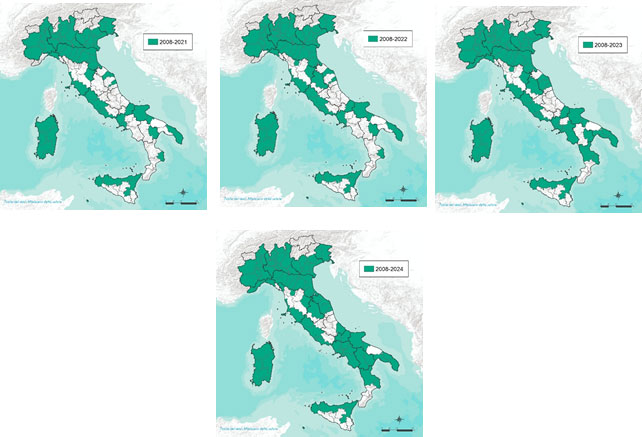
Figure 3c.Distribution of West Nile Virus in Italy (Years 2021-2024).
Starting from 2021, the disease distribution remained largely stable compared to the previous year. In 2022, new outbreaks were reported in Tuscany and Umbria. In 2023, an expansion of the distribution range was observed, involving nearly the entire Marche region (except for the province of Pesaro), most of Campania, and some areas of Calabria. In 2024, further spread was recorded, affecting the entire Campania region, the whole Marche, Molise, Basilicata, one province of Abruzzo, and nearly all of Puglia.
A Multifactorial Problem: The Role of Climate and Human Activities
The epidemiology and transmission cycle of West Nile Virus (WNV) are highly complex and closely linked to both abiotic and biotic factors (18). The emergence and annual spread of West Nile Disease are strongly influenced by abiotic factors such as temperature, precipitation, and environmental conditions, which are also closely linked to land use. Alongside these, biotic factors play a decisive role: host abundance and diversity, vector distribution, and bird migratory routes, all of which facilitate virus circulation. In recent years, climate change and progressive global warming have further intensified these dynamics, promoting the spread of WNV in temperate European countries – including Italy (19) – where the past decade has been marked by recurrent climatic anomalies, with temperatures consistently above average and significant alterations in precipitation patterns (20, 21).
Moreover, Italy lies along migratory routes connecting African wintering areas to breeding grounds in Europe. Therefore, the country is constantly exposed to the risk of introduction of new viral strains. The incidence of the disease may also be influenced by changes in migratory bird behaviour, which are in turn linked to climatic variations (22).
Conclusions and Perspectives: A Future to Monitor
West Nile Virus has now established itself as an endemic-epidemic pathogen in Italy, with its spread reflecting continuously evolving climatic and environmental trends. Twenty years after its first appearance, WND currently represents a significant challenge for both public and veterinary health in the country. The increasing geographical spread and the impact on the most vulnerable population groups highlight the ongoing need to update surveillance and prevention systems.
Within the complex scenario of vector-borne diseases, Italy has developed an integrated strategy that combines veterinary, human, and entomological surveillance, aiming to detect early viral circulation in new areas and promptly activate the necessary measures to limit infection transmission to humans. Such measures include controls over blood transfusions and transplants, as well as direct interventions on vectors.
Surveillance activities, both human and veterinary, are coordinated by the Ministry of Health and supported respectively by the Infectious Diseases Department of the National Institute of Health (Istituto Superiore di Sanità) and the National Reference Center for the study and diagnosis of exotic diseases (CESME) at the Experimental Zooprophylactic Institute of Abruzzo and Molise (Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise), which oversees diagnostic confirmation activities conducted by the respective Territorial Experimental Zooprophylactic Institutes (IIZZSS).
It is also important to promote individual protective behaviors, especially during the warmer months: the use of mosquito repellents, removal of stagnant water collections, and protection of domestic environments with mosquito nets. At the environmental level, many Italian provinces implement seasonal mosquito population control through awareness campaigns, municipal ordinances, larvicidal treatments in drains, and reclamation of at-risk areas. These preventive actions, simple yet fundamental, play a crucial role in reducing virus transmission and protecting public health.
The Italian experience demonstrates that the stable presence of WNV requires a dynamic and multi-level response strategy based on continuous collaboration among public health, veterinary medicine, and environmental management. In a rapidly changing climatic and ecological context, it is essential to maintain high vigilance toward vector-borne viruses by constantly updating scientific knowledge and health policies.
Acknowledgments
We would like to thank Federica Iapaolo for her initial contribution to drafting the article, and Francesca Dall’Acqua and Valentina Zenobio for their invaluable support in the scientific review, content organization, structuring of epidemiological data, which ensured both methodological consistency and scientific accuracy throughout the article.
References
- ECDC https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589.
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550. https://doi.org/10.3390/vaccines80305503. Popescu, C.P.; Florescu, S.A.; Ruta, S.M. West Nile Virus in Central Europe—Pandora’s Box Is Wide Open! Travel Med. Infect. Dis. 2020, 37, 101864.
- Vogels CB, Göertz GP, Pijlman GP, Koenraadt CJ. Vector competence of European mosquitoes for West Nile virus. Emerg Microbes Infect. 2017 Nov 8;6(11):e96. doi: 10.1038/emi.2017.82. PMID: 29116220; PMCID: PMC5717085.
- Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012 Oct;25(4):635-48. doi: 10.1128/CMR.00045-12. PMID: 23034323; PMCID: PMC3485754.
- Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006 Apr;12(4):618-23. doi: 10.3201/eid1204.051379. PMID: 16704810; PMCID: PMC3294705.
- Byas AD, Ebel GD. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens. 2020 Jan 7;9(1):48. doi: 10.3390/pathogens9010048. PMID: 31935992; PMCID: PMC7168622.
- Angenvoort J, Brault AC, Bowen RA, Groschup MH. West Nile viral infection of equids. Vet Microbiol. 2013 Nov 29;167(1-2):168-80. doi: 10.1016/j.vetmic.2013.08.013. Epub 2013 Aug 28. PMID: 24035480; PMCID: PMC4581842.
- Nash, D., Mostashari, F., Fine, A., Miller, J., O'Leary, D., Murray, K., Huang, A., Rosenberg, A., Greenberg, A., Sherman, M., Wong, S., Layton, M., & 1999 West Nile Outbreak Response Working Group (2001). The outbreak of West Nile virus infection in the New York City area in 1999. The New England journal of medicine, 344(24), 1807–1814. https://doi.org/10.1056/NEJM200106143442401
- Koch RT, Erazo D, Folly AJ, et al. Genomic epidemiology of West Nile virus in Europe. One Health. 2023;18:100664. Published 2023 Dec 16. doi:10.1016/j.onehlt.2023.100664
- Nash, D., Mostashari, F., Fine, A., Miller, J., O'Leary, D., Murray, K., Huang, A., Rosenberg, A., Greenberg, A., Sherman, M., Wong, S., Layton, M., & 1999 West Nile Outbreak Response Working Group (2001). The outbreak of West Nile virus infection in the New York City area in 1999. The New England journal of medicine, 344(24), 1807–1814. https://doi.org/10.1056/NEJM200106143442401
- Ziegler, U., Lühken, R., Keller, M., Cadar, D., van der Grinten, E., Michel, F., Albrecht, K., Eiden, M., Rinder, M., Lachmann, L., Höper, D., Vina-Rodriguez, A., Gaede, W., Pohl, A., Schmidt-Chanasit, J., & Groschup, M. H. (2019). West Nile virus epizootic in Germany, 2018. Antiviral research, 162, 39–43. https://doi.org/10.1016/j.antiviral.2018.12.005
- Vlaskamp, D. R., Thijsen, S. F., Reimerink, J., Hilkens, P., Bouvy, W. H., Bantjes, S. E., Vlaminckx, B. J., Zaaijer, H., van den Kerkhof, H. H., Raven, S. F., & Reusken, C. B. (2020). First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 25(46), 2001904. https://doi.org/10.2807/1560-7917.ES.2020.25.46.2001904
- Silverj A, Mencattelli G, Monaco F, et al. Origin and evolution of West Nile virus lineage 1 in Italy. Epidemiol Infect. 2024;152:e150. Published 2024 Dec 2. doi:10.1017/S0950268824001420
- Mencattelli G, Silverj A, Iapaolo F, et al. Epidemiological and Evolutionary Analysis of West Nile Virus Lineage 2 in Italy. Viruses. 2022;15(1):35. Published 2022 Dec 22. doi:10.3390/v15010035
- Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, Lelli R, Murri S, Scicluna MT. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002 Dec;8(12):1372-8. doi: 10.3201/eid0812.020234. PMID: 12498650; PMCID: PMC2738505.
- Rizzo C, Salcuni P, Nicoletti L, Ciufolini MG, Russo F, Masala R, et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012;17(20):20172.
- Marcantonio M, Rizzoli A, Metz M, et al. Identifying the environmental conditions favouring West Nile Virus outbreaks in Europe. PLoS One. 2015;10(3):e0121158. Published 2015 Mar 24. doi:10.1371/journal.pone.0121158
- Magurano F, Remoli ME, Baggieri M, Fortuna C, Marchi A, Fiorentini C, et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin Microbiol Infect. 2012;18(12):E545-7. 10.1111/1469-0691.12018
- Paz S, Semenza JC. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia--a review. Int J Environ Res Public Health. 2013 Aug 9;10(8):3543-62. doi: 10.3390/ijerph10083543. PMID: 23939389; PMCID: PMC3774453.
- Riccardo F, Bella A, Monaco F, Ferraro F, Petrone D, Mateo-Urdiales A, Andrianou XD, Del Manso M, Venturi G, Fortuna C, Di Luca M, Severini F, Caporali MG, Morelli D, Iapaolo F, Pati I, Lombardini L, Bakonyi T, Alexandra O, Pezzotti P, Perrotta MG, Maraglino F, Rezza G, Palamara AT; Italian Arbovirus Surveillance network. Rapid increase in neuroinvasive West Nile virus infections in humans, Italy, July 2022. Euro Surveill. 2022 Sep;27(36):2200653. doi: 10.2807/1560-7917.ES.2022.27.36.2200653. PMID: 36082685; PMCID: PMC9461310.
- Lelli R, Calistri P, Bruno R, Monaco F, Savini G, Di Sabatino D, Corsi I, Pascucci I. West Nile transmission in resident birds in Italy. Transbound Emerg Dis. 2012 Oct;59(5):421-8. doi: 10.1111/j.1865-1682.2011.01287.x. Epub 2011 Dec 28. PMID: 22212727.
Authors (institutional)
- Veterinary Operational Center for Epidemiology, Planning, Information, and Risk Analysis (COVEPI), Istituto Zooprofilattico Sperimentale of Abruzzo and Molise “G. Caporale”
- National Reference Center for Exotic Animal Diseases (CESME), Istituto Zooprofilattico Sperimentale of Abruzzo and Molise “G. Caporale”