Schmallenberg virus - SBVDESCRIPTION OF DISEASE
In late summer 2011 in the German Region of North Rhine-Westphalia non-pathognomonic clinical signs were observed in cattle, namely:
The diagnostic tests excluded the most frequent diseases able to induce a similar clinical picture in cattle.
Metagenomic analysis of blood samples allowed to identify a novel virus provisionally called Schmallenberg virus (SBV) from the German town where it was firstly reported. Etiology
The viral sequencing allowed to classify the virus, previously unknown, within the family Orthobunyavirus in the Simbu serogroup. The serogroup includes 25 viruses, many recognized able to infect ruminants and humans. Orthobunyaviruses are enveloped viruses with a negative sense, ss RNA organized in three segments named Large (L), Medium (M) and Small (S) coding for 5 different proteins. The SBV genome segments show a remarkable similarity with the S segment of Shamonda virus, the M segment of Aino virus and the L segment of Akabane virus addressing the hypothesis of the recombinant origin of the SBV.
The main characteristics of the viruses belonging to the genus Orthobunyavirus are described in the attached table. Epidemiology
The domestic ruminants are susceptible to Schmallenberg virus infection as demonstrated by the detection of the viral genome in tissues of animals belonging to bovine, ovine, caprine and buffaloes species. Antibodies anti-SBV have been demonstrated in a wide number of wild animals (Alpacas, Anatolian water buffalo, Elk, Bison, Red deer, Fallow deer, Roe deer, Sika deer, Muntjac, Chamois, Wild boar and Dogs) as well as in in zoo species.
Even though some Orthobunyavirus affects humans, the zoonotic potential of SBV can be excluded since neither symptoms nor seroconversion were reported in humans.
Field data and experimental studies in EU countries demonstrated the role of biting midges, namely Culicoides, as highly probable vectors of SBV transmission in the infected areas.
Experimental data clarified the role of SBV in causing congenital defects in ruminants (AHS: arthrogryposis hydranencephaly syndrome) due to the capability of the virus to infect the placenta and the umbilical cord reaching the central nervous system (CNS) of the foetuses. Alternative routes of transmission are regarded as unlikely: vertical transmission of SBV can occur in cattle, although the virus has never been isolated neither from blood or skin of newborn animals nor from healthy newborns of affected mothers indicating that further transmission to the vectors is unlikely. A limited number of articles describes the risks of transmission of the virus via semen and embryos. Recent data indicate that SBV may be detected in semen samples with a low frequency (<6 %). However, there is no scientific evidence of transmission through insemination.
Experimental studies showed a short viremia following the infection, 3 to 6 days with a long lasting immune response protective against clinical symptoms. Clinical symptomsClinical signs are related to the age and reproductive status of the animals. Adults usually develop unspecific and transient:
In small ruminants the following symptoms have been reported:
Congenital malformations are observed in foetuses and stillborn animals:
In newborn calves, lambs and kids, it is possible to observe:
The number of herds with SBV confirmed AHS cases compared to the level of infection indicated by seroprevalence studies, suggest that the frequency of clinical disease is low. SBV induces malformed calves only in a very limited number of cases, as demonstrated by experimental infection studies on pregnant cows and ewes. DiagnosisVirus isolation and RT-PCR are the diagnostic tests used to detect the virus or its genome.
The serological diagnosis is based on the ELISA, the Serum neutralization and the immunofluorescence tests.
Once the IIZZSS detect the genome of SBV or the antibodies specific for SBV from clinical specimens, positive samples should be shipped to CESME laboratories for the diagnostic confirmation. Surveillance and disease controlIn Italy following the notification of the first Italian SBV case in February 2012 a national surveillance program was launched (Note of the Italian Ministry of Health 4th of April 2012). A wide spectrum of surveillance actions was put in place to verify the SBV circulation in the area and possibly define the geographical extension of the infection. The surveillance program was mainly based on:
• standardization and re-enforcement of passive surveillance protocols; • risk-based serological surveillance through Serum Neutralization (SN) tests; • entomological surveillance in the involved geographical areas.
As a first step for the re-enforcement of passive surveillance actions, the Italian Ministry of Health adopted clear definitions of suspected and confirmed cases (Table 1).
In case of suspect, several activities should be undertaken, like the census and tracing of animals belonging to the farm where the suspect case was detected and the entomological surveillance in the farm where the suspect case was observed. A risk‑based approach was chosen for the serological surveillance. In case of suspicion, local veterinary services collected blood samples from all animals in the herd/flock, which were then tested by SN at the CESME laboratory. Additional clinical examinations in epidemiologically linked farms and in all animals living within 4 km of radius were also mandatory. A sample of animals was also tested in order to determine the serological prevalence of infection within suspected herds/flocks. The sample size was calculated for an expected within-herd prevalence of 10% and a level of confidence of 95%. This size was chosen according to previous experiences with bluetongue infection in Italy. The suspected and confirmed cases should be communicated by the Official veterinarians to the Italian Ministry of Health (Office III, General Directorate of Animal Health and Veterinary Pharmaceuticals) and registered on the National information system for the notification of Animal Diseases (SIMAN). Once the suspect is confirmed, it is necessary to confirm the case in the SIMAN, and carry out the epidemiological investigation to ascertain the origin of the infection. Furthermore, all the susceptible animals housed in the infected farm should be bled and tested for SBV. 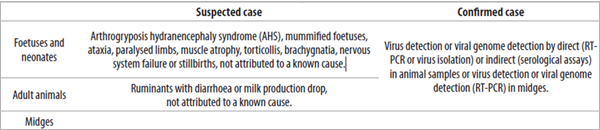
Table 1: Definitions of suspected and confirmed SBV cases adopted by the Italian Ministry of Health. RT-PCR was performed according to the protocol provided by the Friedrich Loeffler Institute (FLI), Germany (Bilk et al., 2012).
© IZSAM October 2016
|
|
Istituto Zooprofilattico Sperimentale
dell'Abruzzo e del Molise "G. Caporale"
Campo Boario | 64100 TERAMO | ITALIA
Telefono 0039.0861.3321 | Fax 0039.0861.332251
e-mail: archivioeprotocollo@izs.it
Posta elettronica certificata: protocollo@pec.izs.it
Partita IVA: 00060330677
Codice Fiscale: 80006470670


